Chemistry:Methylmalonyl-CoA
| |
| Names | |
|---|---|
| Systematic IUPAC name
(9R)-1-[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]-3,5,9-trihydroxy-8,8,20-trimethyl-3,5,10,14,19-pentaoxo-2,4,6-trioxa-18-thia-11,15-diaza-3λ5,5λ5-diphosphahenicosan-21-oic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
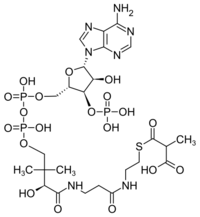
| C25H40N7O19P3S | |
| Molar mass | 867.608 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methylmalonyl-CoA is the thioester consisting of coenzyme A linked to methylmalonic acid. It is an important intermediate in the biosynthesis of succinyl-CoA, which plays an essential role in the tricarboxylic acid cycle (aka the Citric Acid Cycle, or Krebs Cycle).[1] The compound is sometimes referred to as "methylmalyl-CoA".[2]
Biosynthesis and metabolism
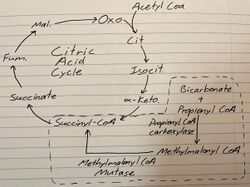
Methylmalonyl-CoA results from the metabolism of fatty acid with an odd number of carbons, of ammino acids valine, isoleucine, methionine, threonine or of cholesterol side-chains, forming Propionyl-CoA.[4] The latter is also formed from propionic acid, which bacteria produce in the intestine.[4] Propionyl-CoA and bicarbonate are converted to Methylmalonyl-CoA by the enzyme propionyl-CoA Carboxylase.[1] It then is converted into succinyl-CoA by methylmalonyl-CoA mutase (MUT). This reaction is a reversible isomerization. In this way, the compound enters the Citric Acid Cycle. The following diagram demonstrates the aforementioned reaction:[2]
Propionyl CoA + Bicarbonate → Methylmalonyl CoA → Succinyl CoA
Vitamin B12
Vitamin B12 plays an integral role in this reaction. Coenzyme B12 (adenosyl-cobalamin) is an organometallic form of Vitamin B12 and serves as the cofactor of Methylmalonyl-CoA mutase, which is an essential enzyme in the human body.[5] The transformation of Methylmalonyl-CoA to Succinyl-CoA by this enzyme is a radical reaction.[5]

Related diseases
Methylmalonic Acidemia (MMA)
This disease occurs when methylmalonyl-CoA mutase is unable to isomerize sufficient amounts of methylmalonyl-CoA into succinyl-CoA.[6] This causes a buildup of propionic and/or methylmalonic acid, which has effects on infants ranging from severe brain damage to death.[4] The disease is linked to Vitamin B12, which is the metabolic precursor to methylmalonyl-CoA mutase.[6][3]
Combined malonic and methylmalonic aciduria (CMAMMA)
In the metabolic disease combined malonic and methylmalonic aciduria (CMAMMA) due to ACSF3 deficiency, methylmalonyl-CoA synthetase is reduced, which converts toxic methylmalonic acid to methylmalonyl-CoA and thus supplies it to the citrate cycle.[7][8] The result is an accumulation of methylmalonic acid.
References
- ↑ 1.0 1.1 "Propionyl-CoA carboxylase - A review". Molecular Genetics and Metabolism 122 (4): 145–152. December 2017. doi:10.1016/j.ymgme.2017.10.002. PMID 29033250.
- ↑ 2.0 2.1 Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, ISBN 0-7167-4339-6
- ↑ 3.0 3.1 "Vitamin B12 , folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation". Journal of Inherited Metabolic Disease 42 (4): 673–685. July 2019. doi:10.1002/jimd.12009. PMID 30693532.
- ↑ 4.0 4.1 4.2 "Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia". Orphanet Journal of Rare Diseases 9 (1): 130. September 2014. doi:10.1186/s13023-014-0130-8. PMID 25205257.
- ↑ 5.0 5.1 5.2 Biochemistry of B12-cofactors in human metabolism. Subcellular Biochemistry. 56. Dordrecht: Springer Netherlands. 2012. pp. 323–346. doi:10.1007/978-94-007-2199-9_17. ISBN 978-94-007-2198-2.
- ↑ 6.0 6.1 "Role of vitamin B12 on methylmalonyl-CoA mutase activity". Journal of Zhejiang University. Science. B 13 (6): 423–437. June 2012. doi:10.1631/jzus.B1100329. PMID 22661206.
- ↑ "Considerations of expanded carrier screening: Lessons learned from combined malonic and methylmalonic aciduria". Molecular Genetics & Genomic Medicine 9 (4): e1621. April 2021. doi:10.1002/mgg3.1621. PMID 33625768.
- ↑ "Role of the malonyl-CoA synthetase ACSF3 in mitochondrial metabolism". Advances in Biological Regulation 71: 34–40. January 2019. doi:10.1016/j.jbior.2018.09.002. PMID 30201289.
 |

