Chemistry:Fullerene whiskers
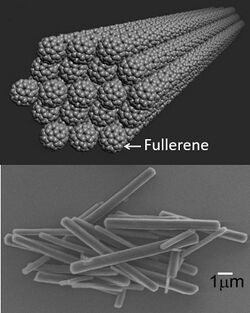
Fullerene whiskers are thin rods composed of fullerene molecules, such as C60, C70, or their mixtures. Hollow fullerene whiskers are called fullerene tubes. Such structures typically have a diameter of a few micrometers. When the diameter becomes smaller than 1 micron, the corresponding structures are called fullerene nanowhiskers or fullerene nanotubes.[1]
Fullerene whiskers and tubes are held together by weak van der Waals forces, and hence are very soft.[1] They can be grown by precipitation at an interface between two liquids. They are semiconductors and have potential uses in field-effect transistors, solar cells, chemical sensors, and photocatalysts. When doped with alkali metals, such as potassium, they become superconductors at 18 K (−255.2 °C; −427.3 °F).[2]
As-grown fullerene nanotubes have hexagonal shapes and face-centered cubic crystal structures. Owing to their relatively large inner diameters (approx. 100 nm) and low reactivity they can accommodate a wide range of nanoparticles. C60 nanotubes decompose upon heating to 416 °C (781 °F) in air.[1]
References
- ↑ 1.0 1.1 1.2 1.3 Miyazawa, K. (2010). "Synthesis and Functions of Fullerene Nanotubes". Inorganic and Metallic Nanotubular Materials. Topics in Applied Physics. 117. pp. 201–214. doi:10.1007/978-3-642-03622-4_15. ISBN 978-3-642-03620-0.
- ↑ 2.0 2.1 Miyazawa, K. (2015). "Synthesis of fullerene nanowhiskers using the liquid–liquid interfacial precipitation method and their mechanical, electrical and superconducting properties". Science and Technology of Advanced Materials 16 (1): 013502. doi:10.1088/1468-6996/16/1/013502. PMID 27877738. Bibcode: 2015STAdM..16a3502M.
 |