Chemistry:Ferrocenecarboxylic acid
From HandWiki
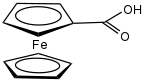
| |
| Names | |
|---|---|
| IUPAC name
Ferrocenecarboxylic acid
| |
| Other names
Ferrocenemonocarboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C11H10FeO2 | |
| Molar mass | 230.044 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.862 g/cm3[1] |
| Melting point | 214–216 °C (417–421 °F; 487–489 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Ferrocenecarboxylic acid is the organoiron compound with the formula (C
5H
5)Fe(C
5H
4CO
2H). It is the simplest carboxylic acid derivative of ferrocene. It can be prepared in two steps from ferrocene by acylation with a 2-chlorobenzoyl chloride followed by hydrolysis.[2]
Reactions and derivatives
The pKa of ferrocenecarboxylic acid is 7.8. The acidity increases more than a thousand-fold, to pH 4.54 upon oxidation to the ferrocenium cation.[3]
By treatment with thionyl chloride, the carboxylic acid anhydride ([(C
5H
5)Fe(C
5H
4CO)]
2O) is produced.[4][5]
Derivatives of ferrocenecarboxylic acid are components of some redox switches.
Related compounds
References
- ↑ Lin, Lily; Berces, Attila; Kraatz, Heinz-Bernhard (1998). "Ferrocenic acid derivatives: Towards Rationalizing Changes in the Electronic and Geometric Structures". Journal of Organometallic Chemistry 556 (1–2): 11–20. doi:10.1016/S0022-328X(97)00785-7.
- ↑ Perry C. Reeves (1977). "Carboxylation of Aromatic Compounds: Ferrocenecarboxylic Acid". Organic Syntheses 56: 28. doi:10.15227/orgsyn.056.0028.
- ↑ Fabbrizzi, Luigi (2020). "The Ferrocenium/Ferrocene Couple: A Versatile Redox Switch". Chemtexts 6 (4). doi:10.1007/s40828-020-00119-6.
- ↑ Tazi, Mehdi; Roisnel, Thierry; Mongin, Florence; Erb, William (2017). "Ferrocenecarboxylic Anhydride: Identification of a New Polymorph". Acta Crystallographica Section C Structural Chemistry 73 (10): 760–766. doi:10.1107/S205322961701124X. PMID 28978780. https://hal-univ-rennes1.archives-ouvertes.fr/hal-01617956/file/Ferrocenecarboxylic%20anhydride%20-HAL.pdf.
- ↑ Lau, Hans; Hart, Harold (1959). "Notes- Preparation and Hydrolysis of Crystalline Ferrocenoyl Chloride". The Journal of Organic Chemistry 24 (2): 280–281. doi:10.1021/jo01084a647.
 |

