Chemistry:Thiosalicylic acid
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Sulfanylbenzoic acid[1] | |||
| Other names
2-Mercaptobenzoic acid
o-Thiosalicylic acid ortho-Thiosalicylic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 508507 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 3838 | |||
| KEGG | |||
| MeSH | 2-Thiosalicylic+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| ortho-C 6H 4(SH)(COOH) | |||
| Molar mass | 154.18 g·mol−1 | ||
| Appearance | Leaf or needle shaped yellow crystals | ||
| Density | 1.49 g cm−3[2] | ||
| Melting point | 162 to 169 °C (324 to 336 °F; 435 to 442 K) | ||
| log P | 2.39 | ||
| Acidity (pKa) | 3.501 | ||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H315, H319, H335 | |||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
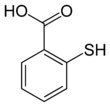
Thiosalicylic acid is an organosulfur compound containing carboxyl and sulfhydryl functional groups. Its molecular formula is ortho-C
6H
4(–SH)(–C(=O)–OH). It is a yellow solid that is slightly soluble in water, ethanol and diethyl ether, and alkanes, but more soluble in DMSO.[3]
Preparation
Thiosalicylic acid can be prepared from anthranilic acid via diazotization followed by the addition of sodium sulfide and then reduction with zinc.[4]
Uses
Thiosalicylic acid is a precursor to the dyestuff thioindigo. It is also used to make the vaccine preservative thiomersal. It is a precursor to drug candidates for treatment of atherosclerosis and melanoma.[5][6] The preservative benzisothiazolinone is prepared from thiosalicylic acid.
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 697. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4. "The prefixes ‘mercapto’ (–SH), and ‘hydroseleno’ or selenyl (–SeH), etc. are no longer recommended."
- ↑ "A13401 Thiosalicylic acid, 98%". Alfa Aesar. http://www.alfa.com/en/GP100W.pgm?DSSTK=A13401&rnd=224806350.
- ↑ Lide, David R., ed (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. p. 3-324. ISBN 978-1-4200-9084-0.
- ↑ C. F. H. Allen; D. D. MacKay (1932). "Thiosalicylic acid". Organic Syntheses 12: 76. doi:10.15227/orgsyn.012.0076.
- ↑ Smalley, Keiran S.M.; Tim G. Eisen (1 April 2002). "Farnesyl thiosalicylic acid inhibits the growth of melanoma cells through a combination of cytostatic and pro-apoptotic effects". International Journal of Cancer 98 (4): 514–522. doi:10.1002/ijc.10213. PMID 11920610.
- ↑ George, Jacob; Arnon Afek; Pnina Keren; Itzhak Herz; Iris Goldberg; Roni Haklai; Yoel Kloog; Gad Keren (2002). "Functional Inhibition of Ras by S-trans,trans-Farnesyl Thiosalicylic Acid Attenuates Atherosclerosis in Apolipoprotein E Knockout Mice". Circulation 105 (20): 2416–2422. doi:10.1161/01.CIR.0000016065.90068.96. PMID 12021230.
 |