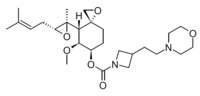
Chemistry:ZGN-1061
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
[(3R,4S,5S,6R)-5-methoxy-4-[(2R,3R)-2-methyl-3-(3-methylbut-2-enyl)oxiran-2-yl]-1-oxaspiro[2.5]octan-6-yl] 3-(2-morpholin-4-ylethyl)azetidine-1-carboxylate | |
| Other names
ZGN-1061, Aclimostat
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C26H42N2O6 | |
| Molar mass | 478.630 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
ZGN-1061 is an experimental drug that was developed by Zafgen for treatment of obesity and type 2 diabetes. It has a similar mechanism of action as the discontinued drug Beloranib but was considered safer; however, its development was also halted because of safety concerns.[1][2][3]
References
- ↑ Christoffersen, Berit Østergaard; Sanchez‐Delgado, Guillermo; John, Linu Mary; Ryan, Donna H.; Raun, Kirsten; Ravussin, Eric (April 2022). "Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss" (in en). Obesity 30 (4): 841–857. doi:10.1002/oby.23374. ISSN 1930-7381. PMC 9310705. https://onlinelibrary.wiley.com/doi/full/10.1002/oby.23374.
- ↑ Wentworth, John M.; Colman, Peter G. (July 2020). "The methionine aminopeptidase 2 inhibitor ZGN‐1061 improves glucose control and weight in overweight and obese individuals with type 2 diabetes: A randomized, placebo‐controlled trial". Diabetes, Obesity and Metabolism 22 (7): 1215–1219. doi:10.1111/dom.14009.
- ↑ Goya Grocin, Andrea; Kallemeijn, Wouter W.; Tate, Edward W. (October 2021). "Targeting methionine aminopeptidase 2 in cancer, obesity, and autoimmunity". Trends in Pharmacological Sciences 42 (10): 870–882. doi:10.1016/j.tips.2021.07.004.
 |

