Chemistry:Fumed silica
Fumed silica (CAS number 112945-52-5), also known as pyrogenic silica because it is produced in a flame, consists of microscopic droplets of amorphous silica fused into branched, chainlike, three-dimensional secondary particles which then agglomerate into tertiary particles. The resulting powder has an extremely low bulk density and high surface area. Its three-dimensional structure results in viscosity-increasing, thixotropic behavior when used as a thickener or reinforcing filler.[1]
Properties
Fumed silica has a very strong thickening effect. Primary particle size is 5–50 nm.[2] The particles are non-porous and have a surface area of 50–600 m2/g. The density is 160–190 kg/m3.
Production
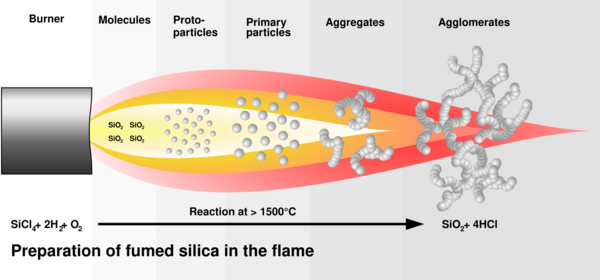
Fumed silica is made from flame pyrolysis of silicon tetrachloride or from quartz sand vaporized in a 3000 °C electric arc.[3] Major global producers are Evonik (who sells it under the name Aerosil), Cabot Corporation (Cab-O-Sil), Wacker Chemie (HDK), Dow Corning, Heraeus (Zandosil), Tokuyama Corporation (Reolosil), OCI (Konasil), Orisil (Orisil) and Xunyuchem(XYSIL).[4]
Applications
Fumed silica serves as a universal thickening agent and an anticaking agent (free-flow agent) in powders. Like silica gel, it serves as a desiccant. It is used in cosmetics for its light-diffusing properties. It is used as a light abrasive, in products like toothpaste. Other uses include filler in silicone elastomer and viscosity adjustment in paints, coatings, printing inks, adhesives and unsaturated polyester resins.[5] Fumed silica readily forms a network structure within bitumen and enhances its elasticity.[6]
Health issues
Fumed silica is not listed as a carcinogen by OSHA, IARC, or NTP. Due to its fineness and thinness, fumed silica can easily become airborne, making it an inhalation hazard capable of causing irritation.
See also
References
- ↑ Flörke, Otto W.; Graetsch, Heribert A.; Brunk, Fred; Benda, Leopold; Paschen, Siegfried; Bergna, Horacio E.; Roberts, William O.; Welsh, William A. et al. (15 April 2008). "Silica". Ullmann's Encyclopedia of Industrial Chemistry: a23_583.pub3. doi:10.1002/14356007.a23_583.pub3. ISBN 978-3527306732.
- ↑ "Fumed Silica". https://www.americanelements.com/fumed-silica-112945-52-5.
- ↑ Garrett, P.R. (1992). Defoaming. Theory and Industrial applications. USA: CRC Press. pp. 239–240. ISBN 0-8247-8770-6.
- ↑ Fumed Silica Manufacturer Overview
- ↑ "Reade Fumed Silica Powder (SiO2)". reade.com. https://www.reade.com/products/fumed-silica-powder-sio2.
- ↑ Zhou, Shengxiong; Li, Siqi; Yan, Chuanqi (2023-09-15). "Influence of fumed silica nanoparticles on the rheological and anti-aging properties of bitumen". Construction and Building Materials 397: 132388. doi:10.1016/j.conbuildmat.2023.132388. ISSN 0950-0618. https://www.sciencedirect.com/science/article/pii/S0950061823021049.
 |