Chemistry:Terbium acetylacetonate
From HandWiki
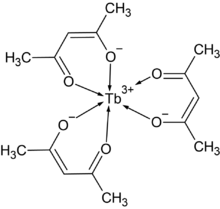
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C15H21O6Tb | |
| Molar mass | 456.252 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Terbium acetylacetonate is a coordination compound with the chemical formula Tb(C5H7O2)3, or Tb(acac)3 for short. It can be prepared by the reaction of ammonia, acetylacetone and terbium nitrate:[1]
- 3 NH3 + 3 Hacac + Tb(NO3)3 → Tb(acac)3 + 3 NH4NO3
It reacts with 5-[(2-thiophene methylene)amino]-8-hydroxyquinoline (L) by heating in acetonitrile/dichloromethane solution to obtain light yellow [Tb(acac)4(L)6(μ3-OH)2]·CH3CN crystals.[2] It can be used in the preparation of some optical materials.[3][4][5]
References
- ↑ Anees A. Ansari, Joselito P. Labis (2012). "One-pot synthesis and photoluminescence properties of luminescent functionalized mesoporous SiO2@Tb(OH)3 core–shell nanospheres" (in en). Journal of Materials Chemistry 22 (32): 16649. doi:10.1039/c2jm33583b. ISSN 0959-9428. http://xlink.rsc.org/?DOI=c2jm33583b. Retrieved 2021-09-20.
- ↑ Wen-Min Wang, Lei Huai, Xi-Wen Wang, Kai-Jun Jiang, Hai-Yun Shen, Hong-Ling Gao, Ming Fang, Jian-Zhong Cui (2020). "Structures, magnetic refrigeration and single molecule-magnet behavior of five rhombus-shaped tetranuclear Ln( iii )-based clusters" (in en). New Journal of Chemistry 44 (25): 10266–10274. doi:10.1039/D0NJ01969K. ISSN 1144-0546. http://xlink.rsc.org/?DOI=D0NJ01969K. Retrieved 2021-09-20.
- ↑ Rui Jia, Ting Gao, Yu Yang, Wenbin Sun, Ruoxi Chen, Pengfei Yan, Guangfeng Hou (Sep 2015). "Luminescence of Salen Lanthanide Bimetallic Complexes: Dual Emission and Energy Transfer: Luminescence of Salen Lanthanide Bimetallic Complexes" (in en). Zeitschrift für anorganische und allgemeine Chemie 641 (11): 1974–n/a. doi:10.1002/zaac.201500138. https://onlinelibrary.wiley.com/doi/10.1002/zaac.201500138. Retrieved 2021-09-20.
- ↑ Gaël Zucchi, Taewoo Jeon, Denis Tondelier, Dmitry Aldakov, Pierre Thuéry, Michel Ephritikhine, Bernard Geffroy (2010). "White electroluminescence of lanthanide complexes resulting from exciplex formation" (in en). Journal of Materials Chemistry 20 (11): 2114. doi:10.1039/b921740a. ISSN 0959-9428. http://xlink.rsc.org/?DOI=b921740a. Retrieved 2021-09-20.
- ↑ Anna M. Kaczmarek, Ying‐Ya Liu, Mariusz K. Kaczmarek, Hengshuo Liu, Flavia Artizzu, Luís D. Carlos, Pascal Van Der Voort (2020-01-27). "Developing Luminescent Ratiometric Thermometers Based on a Covalent Organic Framework (COF)" (in en). Angewandte Chemie International Edition 59 (5): 1932–1940. doi:10.1002/anie.201913983. ISSN 1433-7851. PMID 31777996. https://onlinelibrary.wiley.com/doi/10.1002/anie.201913983. Retrieved 2021-09-20.
 |

