Chemistry:Gentianine
From HandWiki
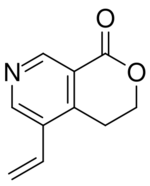
| |
| Names | |
|---|---|
| IUPAC name
5-Ethenyl-3,4-dihydropyrano[3,4-c]pyridin-1-one
| |
| Other names
Erythricine
| |
| Identifiers | |
3D model (JSmol)
|
|
| 137011 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H9NO2 | |
| Molar mass | 175.187 g·mol−1 |
| Melting point | 82–83 °C (180–181 °F; 355–356 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Gentianine is a pyridine-derived alkaloid. Originally isolated in 1944 from Gentiana kirilowi,[2] it has also been found in Gentiana macrophylla,[3] fenugreek,[4] Strychnos angolensis,[5] Strychnos xantha,[5] and other plants.[1]
Gentianine is a crystalline solid with a melting point of 82-83 °C.[1] It is a base that forms salts, such as the hydrochloride salt, upon treatment with acids.[1]
Gentianine has been studied for its potential anti-inflammatory properties.[3][6][7][8]
References
- ↑ 1.0 1.1 1.2 1.3 Merck Index (11th ed.). 4284 Gentianine.
- ↑ Proskurnina (1944), J. Gen. Chem. USSR, 14, 1148.
- ↑ 3.0 3.1 Kwak, Wie-Jong; Kim, Joo-Hyon; Ryu, Keun-Ho; Cho, Yong-Baik; Jeon, Sun-Duck; Moon, Chang-Kiu (2005). "Effects of Gentianine on the Production of Pro-inflammatory Cytokines in Male Sprague-Dawley Rats Treated with Lipopolysaccharide (LPS)". Biological and Pharmaceutical Bulletin 28 (4): 750–753. doi:10.1248/bpb.28.750. PMID 15802824.
- ↑ "Gentianine (FDB007359)". FooDB. The Metabolomics Innovation Centre. https://foodb.ca/compounds/FDB007359.
- ↑ 5.0 5.1 "Gentianine". PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Gentianine.
- ↑ Wenjin, Chen; Jianwei, Wang (2017). "Protective Effect of Gentianine, a compound from du Huo Ji Sheng Tang, against Freund's Complete Adjuvant-Induced Arthritis in Rats". Inflammation 40 (4): 1401–1408. doi:10.1007/s10753-017-0583-8. PMID 28501981.
- ↑ Wang, Na; Liu, Yao; Jia, Caixia; Gao, Chengwen; Zheng, Ting; Wu, Mingxuan; Zhang, Qian; Zhao, Xiangzhong et al. (2021). "Machine learning enables discovery of Gentianine targeting TLR4/NF-κB pathway to repair ischemic stroke injury". Pharmacological Research 173: 105913. doi:10.1016/j.phrs.2021.105913. PMID 34563661.
- ↑ Mirzaee, Fatemeh; Hosseini, Amirsaeed; Jouybari, Hossein Bakhshi; Davoodi, Ali; Azadbakht, Mohammad (2017). "Medicinal, biological and phytochemical properties of Gentiana species". Journal of Traditional and Complementary Medicine 7 (4): 400–408. doi:10.1016/j.jtcme.2016.12.013. PMID 29034186.
 |

