Chemistry:Thiopyran
From HandWiki
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2H-Thiopyran; 4H-Thiopyran
| |||
| Identifiers | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII |
| ||
| Properties | |||
| C5H6S | |||
| Molar mass | 98.16 g·mol−1 | ||
| Density | 1.1446g/cm3 | ||
| Boiling point | 241.5 °C (466.7 °F; 514.6 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
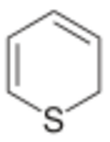
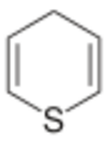
Thiopyran is a heterocyclic compound with the chemical formula C5H6S.[1] It has two isomers, 2H-thiopyran and 4H-thiopyran, which differ by the location of double bonds. Thiopyrans are analogous to pyrans in which the oxygen atoms have been replaced by sulfur atoms.
See also
- Thiopyrylium
- Thio-
References
- ↑ Kuthan, J.; Šcebek, P.; Böuhm, S. (1994). Developments in the Chemistry of Thiopyrans, Selenopyrans, and Teluropyrans. Advances in Heterocyclic Chemistry. 59. pp. 179–244. doi:10.1016/S0065-2725(08)60008-2. ISBN 9780120207596.
 |