Chemistry:Fludioxonil
| |

| |
| Names | |
|---|---|
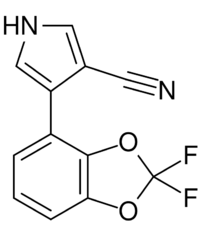
| Preferred IUPAC name
4-(2,2-Difluoro-2H-1,3-benzodioxol-4-yl)-1H-pyrrole-3-carbonitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H6F2N2O2 | |
| Molar mass | 248.189 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fludioxonil is a synthetic phenylpyrrole chemical introduced by Ciba-Geigy (now Syngenta) in 1993 for use as a non-systemic fungicide. It is a structural analog of the natural fungicide pyrrolnitrin.
It is used for the treatment of crops, particularly cereals, fruits and vegetables, and ornamental plants. It is often used in combination with another fungicide such as Cyprodinil. There was a particularly bad crop failure due to multiresistant B. cinerea in strawberry in Florida in 2012; in that year and many other years, fludioxonil is the only fungicide still providing any protection.[1]
Its mode of action is to inhibit transport-associated phosphorylation of glucose, which reduces mycelial growth rate.[2] Fludioxonil is used against Fusarium, Rhizoctonia, Alternaria, Botrytis cinerea, and Stromatinia cepivora.
Brand names include seed treatments: Celest, Agri Star Fludioxonil 41 ST, Dyna-shield Fludioxonil, Maxim 4 FS, and Spirato 480 FS, as well as foliar applications: Switch (fludioxonil + cyprodinil).[3]
Environmental and Health hazards
It is toxic to fish and other aquatic organisms.[4] It has been detected as a residue in baby foods.[5]
See also
References
- ↑
- Hahn, Matthias (2014-05-28). "The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study". Journal of Chemical Biology (Springer) 7 (4): 133–141. doi:10.1007/s12154-014-0113-1. ISSN 1864-6158. PMID 25320647.
- Mari, Marta; Di francesco, Alessandra; Bertolini, Paolo (2014). "Control of fruit postharvest diseases: old issues and innovative approaches". Stewart Postharvest Review (Stewart Postharvest Solutions) 10 (1): 1–4. doi:10.2212/spr.2014.1.1. ISSN 1745-9656. http://www.ingentaconnect.com/content/sphs/sphr/2014/00000010/00000001/art00001.
- Amiri, A.; Heath, S. M.; Peres, N. A. (2013). "Phenotypic Characterization of Multifungicide Resistance in Botrytis cinerea Isolates from Strawberry Fields in Florida". Plant Disease (American Phytopathological Society) 97 (3): 393–401. doi:10.1094/pdis-08-12-0748-re. ISSN 0191-2917. PMID 30722364.
- ↑ "Fludioxonil (Ref: CGA 173506)". http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/330.htm.
- ↑ "What's on your seed?". Integrated Pest and Crop Management, University of Wisconsin-Madison. https://ipcm.wisc.edu/download/pubsPM/Whats_on_your_seed_web.pdf.
- ↑ Paranjape, Kalyani, Vasant Gowariker, V. N. Krishnamurthy, and Sugha Gowariker. The Pesticide Encyclopedia. CABI, 2014.
- ↑ Evans, Sydney; Lacey, Anthony (November 15, 2023). "Pesticides still found in baby food, but biggest toxic threats eliminated". Environmental Working Group. https://www.ewg.org/research/pesticides-still-found-baby-food-biggest-toxic-threats-eliminated.
 |


