Chemistry:Bouveault–Blanc reduction
| Bouveault-Blanc reduction | |
|---|---|
| Named after | Louis Bouveault Gustave Louis Blanc |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | bouveault-blanc-reduction |
| RSC ontology ID | RXNO:0000119 |
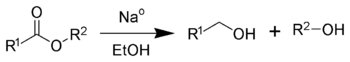
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal.[1] It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903.[2][3][4] Bouveault and Blanc demonstrated the reduction of ethyl oleate and n-butyl oleate to oleyl alcohol.[5] Modified versions of which were subsequently refined and published in Organic Syntheses.[6][7][8]
This reaction is used commercially although for laboratory scale reactions it was made obsolete by the introduction of lithium aluminium hydride.[1]
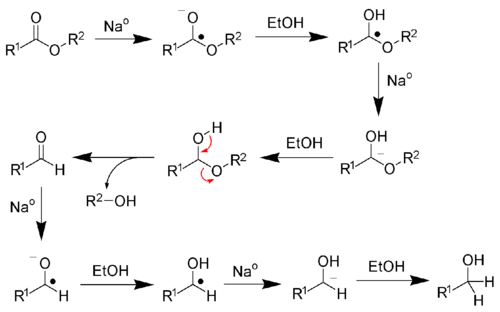
Reaction mechanism
Sodium metal is a one-electron reducing agent. Four equivalents of sodium are required to fully reduce each ester. Ethanol serves as a proton source.[1] The reaction produces sodium alkoxides, according to the following stoichiometry:
- RCOOR' + 6 Na + 4 CH3CH2OH → RCH2ONa + R'ONa + 4 CH3CH2ONa
In practice, considerable sodium is consumed by the formation of hydrogen. For this reason, an excess of sodium is often required. Because the hydrolysis of sodium is rapid, not to mention dangerous, the Bouveault-Blanc reaction requires anhydrous ethanol.[9][8] The mechanism of the reaction follows:[1]
Consistent with this mechanism, sodium-ethanol mixtures will also reduce ketones to alcohols.[10]
This approach to reducing esters was widely used prior to the availability of hydride reducing agents such as lithium aluminium hydride and related reagents. It requires vigorous reaction conditions and has a significant risk of fires, explaining its relative unpopularity. One modification involves encapsulating the alkali metal into a silica gel, which has a safety and yield profile similar to that of hydride reagents.[11] Another modification uses a sodium dispersion.[12][13]
See also
- Acyloin condensation – The reductive coupling of esters, using sodium, to yield an α-hydroxyketone
- Akabori amino-acid reaction – The reduction of amino acid esters, by sodium, to yield aldehydes
- Birch reduction – For the reduction of alkenes using sodium
- Bouveault aldehyde synthesis – Another organometallic reaction by Bouveault where a Grignard reagent is converted to an aldehyde
References
- ↑ 1.0 1.1 1.2 1.3 Wang, Zerong, ed (2009). "109. Bouveault–Blanc Reduction". Comprehensive Organic Name Reactions and Reagents. pp. 493–496. doi:10.1002/9780470638859.conrr109. ISBN 978-0-471-70450-8.
- ↑ Bouveault, L.; Blanc, G. (1903). "Préparation des alcools primaires au moyen des acides correspondants" (in French). Compt. Rend. 136: 1676–1678. http://gallica.bnf.fr/ark:/12148/bpt6k3091c/f1676.image.langFR.
- ↑ Bouveault, L.; Blanc, G. (1903). "Préparation des alcools primaires au moyen des acides correspondants" (in French). Compt. Rend. 137: 60–62.
- ↑ Bouveault, L.; Blanc, G. (1904). "Transformation des acides monobasiques saturés dans les alcools primaires correspondants" (in French). Bull. Soc. Chim. Fr. 31: 666–672. http://gallica.bnf.fr/ark:/12148/bpt6k5469971k/f670.image.
- ↑ Bouveault, L.; Blanc, G. (1904). "Hydrogénation des éthers des acides possédant en outre les fonctions éther-oxyde ou acétal" (in French). Bull. Soc. Chim. Fr. 31 (3): 1210–1213. http://gallica.bnf.fr/ark:/12148/bpt6k5469971k/f1214.image.
- ↑ Reid, E. E.; Cockerille, F. O.; Meyer, J. D.; Cox, W. M.; Ruhoff, J. R. (1935). "Oleyl Alcohol". Organic Syntheses 15: 51. doi:10.15227/orgsyn.015.0051.
- ↑ Adkins, Homer; Gillespie, R. H. (1949). "Oleyl alcohol". Organic Syntheses 29: 80. doi:10.15227/orgsyn.029.0080.
- ↑ 8.0 8.1 Ford, S. G.; Marvel, C. S. (1930). "Lauryl Alcohol". Organic Syntheses 10: 62. doi:10.15227/orgsyn.010.0062.
- ↑ R. H. Manske (1934). "Decamethylene Glycol". Organic Syntheses 14: 20. doi:10.15227/orgsyn.014.0020.
- ↑ Whitmore, Frank C.; Otterbacher, T. (1930). "2-Heptanol". Organic Syntheses 10: 60. doi:10.15227/orgsyn.010.0060.
- ↑ Bodnar, Brian S.; Vogt, Paul F. (2009). "An Improved Bouveault-Blanc Ester Reduction with Stabilized Alkali Metals". J. Org. Chem. 74 (6): 2598–2600. doi:10.1021/jo802778z. PMID 19219971.
- ↑ An, Jie; Work, D. Neil; Kenyon, Craig; Procter, David J. (2014). "Evaluating a Sodium Dispersion Reagent for the Bouveault–Blanc Reduction of Esters". J. Org. Chem. 79 (14): 6743–6747. doi:10.1021/jo501093g. PMID 24941291.
- ↑ Han, Minhui; Ma, Xiaodong; Yao, Shangchu; Ding, Yuxuan; Yan, Zihan; Adijiang, Adila; Wu, Yufei; Li, Hengzhao et al. (2017). "Development of a Modified Bouveault–Blanc Reduction for the Selective Synthesis of α,α-Dideuterio Alcohols". J. Org. Chem. 82 (2): 1285–1290. doi:10.1021/acs.joc.6b02950. PMID 28029787.
External links
 |