Chemistry:Primary alcohol
From HandWiki
Short description: Alcohol in which the hydroxy group is bonded to a primary carbon atom
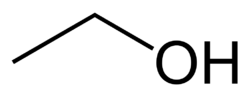
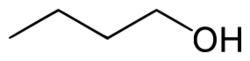
A primary alcohol is an alcohol in which the hydroxy group is bonded to a primary carbon atom. It can also be defined as a molecule containing a “–CH2OH” group.[1] In contrast, a secondary alcohol has a formula “–CHROH” and a tertiary alcohol has a formula “–CR2OH”, where “R” indicates a carbon-containing group.
Examples of primary alcohols include ethanol and 1-butanol.
Methanol is also generally regarded as a primary alcohol,[2][3] including by the 1911 edition of the Encyclopædia Britannica.[4]
See also
- Alcohol (especially Nomenclature section for discussion on Secondary and Tertiary alcohols.)
- Oxidation of primary alcohols to carboxylic acids
References
- ↑ "Definition: primary alcohol from Online Medical Dictionary". http://cancerweb.ncl.ac.uk/cgi-bin/omd?primary+alcohol. [yes|permanent dead link|dead link}}]
- ↑ "Introducing Alcohols". Jim Clark (2015). http://www.chemguide.co.uk/organicprops/alcohols/background.html.
- ↑ Albert S. Tarendash (2001). Let's review: chemistry, the physical setting. Boston, Mass: Barron's. p. 161. ISBN 0-7641-1664-9. https://archive.org/details/letsreviewchemis03edtare/page/161.
- ↑ Chisholm, Hugh, ed (1911). "Alcohols". Encyclopædia Britannica. 1 (11th ed.). Cambridge University Press. p. 527.
 |
