Chemistry:Vinyl neodecanoate
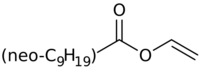
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethenyl 7,7-dimethyloctanoate | |
| Other names
Neodecanoic acid vinyl ester; VeoVa 10
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3082 |
| |
| |
| Properties | |
| C12H22O2 | |
| Molar mass | 198.306 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Density | 0.882 g/mL[2] |
| Boiling point | 60–216 °C (140–421 °F; 333–489 K)[2] |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H410 | |
| P273, P391, P501 | |
| Flash point | 83 °C; 182 °F; 356 K[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Vinyl neodecanoate (trade name VeoVa 10) is a vinylic monomer that is virtually always used in combination with other monomers to create lattices or emulsion polymers.[3] The trade name is an acronym of Vinyl ester of Versatic Acid with the number 10 meaning 10 carbons in the molecule. It has a medium to low glass transition temperature of -3 °C. Chemically, it is a mixture of isomeric vinyl esters of neodecanoic acid.
Uses
Vinyl neodecanoate is mainly used as a modifying monomer in conjunction with other monomers and particularly the manufacture of vinyl acetate based polymer emulsions by the process of emulsion polymerization.[4] Vinyl neodecanoate-containing polymers are used in decorative emulsion paints, plasters and renders especially in Europe.[5] Vinyl neodecanoate, like most vinyl ester monomers, is very hydrophobic and the structure is highly branched with a tertiary substituted α-carbon. It is used as a hydrophobic co-monomer. This structure renders the polymers produced from it, very resistant to alkali degradation as there is no hydrogen (thus proton producing species) on the α-carbon.[6] They have good resistance to degradation from ultraviolet light.[7] The monomer has even been used to produce vibration dampening resins.[8] It is claimed to produce coatings with high liquid stain repellency.[9]
See also
References
- ↑ 1.0 1.1 "Neodecanoic acid vinyl ester". caslab.com. http://www.caslab.com/Neodecanoic_acid_vinyl_ester_CAS_51000-52-3.
- ↑ 2.0 2.1 "Vinyl neodecanoate, mixture of isomers". Sigma-Aldrich. https://www.sigmaaldrich.com/catalog/product/aldrich/134481.
- ↑ "Technical Data Sheet - VeoVa™ 10 Monomer". http://www.neochemical.ru/File/TDS_VeoVa_10.pdf.
- ↑ "VeoVa™ 10 - Hexion - datasheet" (in en). https://coatings.specialchem.com/product/m-hexion-veova-10.
- ↑ "VeoVa 10 vinyl ester based binders for interior silk and exterior architectural paints" (in en). https://www.chemarc.com/content/veova-10-vinyl-ester-based-binders-for-interior-silk-and-exterior-architectural-paints/59019a0784583c4676851992.
- ↑ "Vinyl Acetate-Versatic Acid Vinyl Ester Copolymer for Masonry Coatings" (in en-US). https://www.paint.org/ct-archives/vinyl-acetate-versatic-acid-vinyl-ester-copolymer-for-masonry-coatings/.
- ↑ "Branched Vinyl Ester Monomers" (in en). https://www.pcimag.com/articles/98417-branched-vinyl-ester-monomers.
- ↑ Taniuchi, Mamoru; Takatsuka, Kohro; Fujiwara, Haruo; Korida, Kazuhiko (1991-03-01). "Development of vibration-damping resins for room-temperature application" (in en). Metallurgical Transactions A 22 (3): 629–631. doi:10.1007/BF02670284. ISSN 1543-1940. Bibcode: 1991MTA....22..629T.
- ↑ Rohm and Haas (2015-04-16). "Coating composition with improved liquid stain repellency World Patent WO 2015/051514 A1". https://patentimages.storage.googleapis.com/8c/f8/e9/5ba88fce04ba2c/WO2015051514A1.pdf.
External links
 |

