Chemistry:2-Iodobenzoic acid
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Iodobenzoic acid | |
| Other names
o-Iodobenzoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
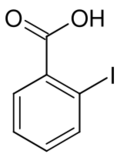
| C7H5IO2 | |
| Molar mass | 248.018 g/mol |
| Appearance | white solid |
| Density | 2.25 g/cm3 |
| Melting point | 162 °C (324 °F; 435 K) |
| Related compounds | |
Related compounds
|
4-Iodobenzoic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
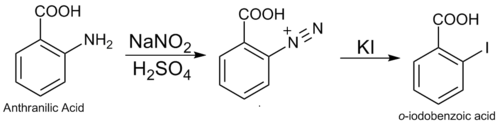
2-Iodobenzoic acid, or o-iodobenzoic acid, is an isomer of iodobenzoic acid.[1] The synthesis of 2-iodobenzoic acid via the diazotization of anthranilic acid is commonly performed in university organic chemistry labs. One of its most common uses is as a precursor for the preparation of IBX and Dess–Martin periodinane, both used as mild oxidants.
Synthesis
2-Iodobenzoic acid can be synthesized by a Sandmeyer reaction: the diazotization of anthranilic acid followed by a reaction with iodide.
See also
References
 |