Chemistry:Bryostatin
| |
| Names | |
|---|---|
| IUPAC name
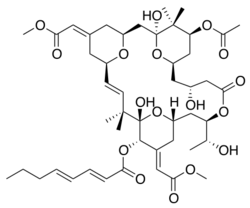
(1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,20R,23R,25S)-25-Acetoxy-1,11,20-trihydroxy-17-[(1R)-1-hydroxyethyl]-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo[21.3.1.13,7.111,15]nonacos-8-en-12-yl (2E,4E)-2,4-octadienoate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| KEGG |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C47H68O17 | |
| Molar mass | 905.044 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bryostatins are a group of macrolide lactones from (bacterial symbionts of) the marine organism Bugula neritina that were first collected and provided to JL Hartwell’s anticancer drug discovery group at the National Cancer Institute (NCI) by Jack Rudloe.[1] Bryostatins are potent modulators of protein kinase C. They have been studied in clinical trials as anti-cancer agents, as anti-AIDS/HIV agents and in people with Alzheimer's disease.
Biological effects
Bryostatin 1 is a potent modulator of protein kinase C (PKC).[2]
It showed activity in laboratory tests in cells and model animals, so it was brought into clinical trials. As of 2014 over thirty clinical trials had been conducted, using bryostatin alone and in combination with other agents, in both solid tumors and blood tumors; it did not show a good enough risk:benefit ratio to be advanced further.[3]
It showed enough promise in animal models of Alzheimer's disease that a Phase II trial was started by 2010;[4] the trial was sponsored by the Blanchette Rockefeller Neurosciences Institute.[5] Scientists from that institute started a company called Neurotrope,[6] and launched another clinical trial in Alzheimer's disease,[7] preliminary results of which were released in 2017. [8][9]
Bryostatin has also been studied in people with HIV.[2]
Chemistry
Bryostatin 1 was first isolated in the 1960s by George Pettit from extracts of a species of bryozoan, Bugula neritina, based on research from samples originally provided by Jack Rudloe to Jonathan L. Hartwell’s anticancer drug discovery group at the National Cancer Institute (NCI).[1] The structure of bryostatin 1 was determined in 1982.[10] As of 2010 20 different bryostatins had been isolated.[11]
The low concentration in bryozoans (to extract one gram of bryostatin, roughly one tonne of the raw bryozoans is needed) makes extraction unviable for large scale production. Due to the structural complexity, total synthesis has proved difficult, with only a few total syntheses reported so far. Total syntheses have been published for bryostatins 1, 2, 3, 7, 9 and 16.[12][13][14][15][16][17][18][19] Among them, Wender’s total synthesis of bryostatin 1 [19] is the shortest synthesis of any bryostatin reported, to date.
A number of structurally simpler synthetic analogs also have been prepared which exhibit similar biological profile and in some cases greater potency, which may provide a practical supply for clinical use.[20]
Biosynthesis
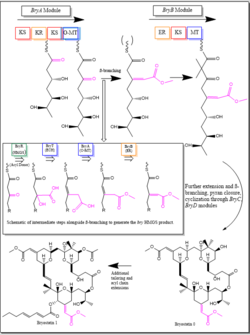
In B. Neritina, bryostatin biosynthesis is carried out through a type I polyketide synthase cluster, bry. BryR is the secondary metabolism homolog of HMG-CoA synthase, which is the PKS in bacterial primary metabolism. In the bryostatin pathway, the BryR module catalyzes β-Branching between a local acetoacetyl acceptor acyl carrier protein (ACP-a) and an appropriate donor BryU acetyl-ACP (ACP-d).[21]
The first step involves the loading of a malonyl unit onto a discrete BryU ACP-d within an initial BryA module. The extended BryU product in BryA is then loaded onto a cysteine sidechain of BryR for interaction with ACP-a. Upon interaction, BryR then catalyzes β-Branching, facilitating an aldol reaction between the alpha-carbon of the BryU unit and the β-ketone of ACP-a, yielding a product similar to HMGS products in primary metabolism. After β-Branching, subsequent dehydration by a BryT enoyl-CoA hydratase homolog (ECH), as well as BryA O-methylation and BryB double bond isomerization of the generated HMGS product, are carried out in specific domains of the bry cluster. These post-β-Branching steps generate the vinyl methylester moieties which are found in all natural product bryostatins. Finally, BryC and BryD are responsible for further extension, pyran ring closure, and cyclization of the HMGS product to produce the novel bryostatin product.[22]
In the presence of BryR, ACP-d conversion to holo-ACP-d was observed prior to β-Branching. BryR was shown to have high specificity for ACP-d only after this conversion. Specificity for these protein-bound groups is a feature that differentiates the HMGS homologs found in primary metabolism, where HMGS typically acts on substrates linked to Coenzyme A, from those found in non-ribosomal peptide synthase (NRPS) or PKS pathways such as the bryostatin pathway.[21]
References
- ↑ 1.0 1.1 "The Bryostatins' Tale". Chemical and Engineering News 89 (43): 10–17. 24 October 2011. doi:10.1021/cen-v089n043.p010. https://pubs.acs.org/cen/coverstory/89/8943cover.html.
- ↑ 2.0 2.1 "Marine natural products: bryostatins in preclinical and clinical studies". Pharmaceutical Biology 52 (2): 237–42. February 2014. doi:10.3109/13880209.2013.804100. PMID 24033119.
- ↑ name=Kollar2014rev>"Marine natural products: bryostatins in preclinical and clinical studies". Pharmaceutical Biology 52 (2): 237–42. February 2014. doi:10.3109/13880209.2013.804100. PMID 24033119.
- ↑ "Bryostatins: biological context and biotechnological prospects". Current Opinion in Biotechnology 21 (6): 834–42. December 2010. doi:10.1016/j.copbio.2010.09.018. PMID 20971628.
- ↑ Clinical trial number NCT00606164 for "Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics Study of Bryostatin 1 in Patients With Alzheimer's Disease" at ClinicalTrials.gov
- ↑ "Alzheimer's Researchers Discover Bryostatin Can Slow, Reverse Disease Progression". Alzheimer's News Today. 19 August 2014. https://alzheimersnewstoday.com/2014/08/19/alzheimers-researchers-discover-bryostatin-can-slow-reverse-disease-progression/.
- ↑ Clinical trial number NCT02431468 for "A Study Assessing Bryostatin in the Treatment of Moderately Severe to Severe Alzheimer's Disease" at ClinicalTrials.gov
- ↑ Taylor, Nick Paul (May 1, 2017). "Neurotrope misses primary endpoint in Alzheimer's trial". FierceBiotech. http://www.fiercebiotech.com/biotech/neurotrope-misses-primary-endpoint-alzheimer-s-trial.
- ↑ "Bryostatin Effects on Cognitive Function and PKCɛ in Alzheimer's Disease Phase IIa and Expanded Access Trials". Journal of Alzheimer's Disease 58 (2): 521–535. 2017. doi:10.3233/JAD-170161. PMID 28482641.
- ↑ "Isolation and structure of bryostatin 1". J. Am. Chem. Soc. 104 (24): 6846–6848. 1982. doi:10.1021/ja00388a092.
- ↑ "New approaches to the total synthesis of the bryostatin antitumor macrolides". Chemistry: An Asian Journal 5 (4): 704–54. April 2010. doi:10.1002/asia.200900634. PMID 20354984.
- ↑ "Total synthesis of bryostatin 1". Journal of the American Chemical Society 133 (4): 744–7. February 2011. doi:10.1021/ja110198y. PMID 21175177.
- ↑ "Total Synthesis of Bryostatin 2". J. Am. Chem. Soc. 121 (33): 7540–7552. 1999. doi:10.1021/ja990860j.
- ↑ "Total Synthesis of Bryostatin". Angewandte Chemie 39 (13): 2290–2294. July 2000. doi:10.1002/1521-3773(20000703)39:13<2290::AID-ANIE2290>3.0.CO;2-6. PMID 10941067.
- ↑ "Synthesis of Bryostatin 7". J. Am. Chem. Soc. 112 (20): 7407–7408. 1990. doi:10.1021/ja00176a058.
- ↑ "Total synthesis of bryostatin 7 via C-C bond-forming hydrogenation". Journal of the American Chemical Society 133 (35): 13876–9. September 2011. doi:10.1021/ja205673e. PMID 21780806.
- ↑ "Total synthesis of bryostatin 9". Journal of the American Chemical Society 133 (24): 9228–31. June 2011. doi:10.1021/ja203034k. PMID 21618969.
- ↑ "Total synthesis of bryostatin 16 using atom-economical and chemoselective approaches". Nature 456 (7221): 485–8. November 2008. doi:10.1038/nature07543. PMID 19037312. Bibcode: 2008Natur.456..485T.
- ↑ 19.0 19.1 "Scalable synthesis of bryostatin 1 and analogs, adjuvant leads against latent HIV". Science 358 (6360): 218–223. October 2017. doi:10.1126/science.aan7969. PMID 29026042.
- ↑ "The practical synthesis of a novel and highly potent analogue of bryostatin". Journal of the American Chemical Society 124 (46): 13648–9. November 2002. doi:10.1021/ja027509+. PMID 12431074.
- ↑ 21.0 21.1 "Polyketide β-branching in bryostatin biosynthesis: identification of surrogate acetyl-ACP donors for BryR, an HMG-ACP synthase". Chemistry & Biology 17 (10): 1092–100. October 2010. doi:10.1016/j.chembiol.2010.08.008. PMID 21035732.
- ↑ "Chemoenzymatic Dissection of Polyketide β-Branching in the Bryostatin Pathway". Marine Enzymes and Specialized Metabolism - Part A. Methods in Enzymology. 604. 2018. pp. 207–236. doi:10.1016/bs.mie.2018.01.034. ISBN 9780128139592.
Further reading
- "Drugs from the seas - current status and microbiological implications". Applied Microbiology and Biotechnology 59 (2–3): 125–34. July 2002. doi:10.1007/s00253-002-1006-8. PMID 12111137.
External links
- Kilham, Chris. "The Importance of Drugs From the Sea". Fox News Health. http://health.foxnews.mobi/quickPage.html?page=31737&content=60866907&pageNum=-1.
- "Bryostatin 1". Aphios Corporation. http://aphios.com/products/research-chemicals/bryostatin-1.html.
- "Bryostatin 2". Aphios Corporation. http://aphios.com/products/research-chemicals/bryostatin-2.html.
- "Bryostatin 3". Aphios Corporation. http://aphios.com/products/research-chemicals/bryostatin-3.html.
 |