Chemistry:Radium iodate
From HandWiki
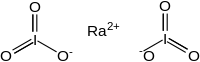
| |
| Names | |
|---|---|
| Preferred IUPAC name
Radium iodate | |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| I2O6Ra | |
| Molar mass | 576 g·mol−1 |
| Appearance | colorless solid |
| poorly soluble | |
| Related compounds | |
Related compounds
|
Barium iodate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Radium iodate is an inorganic compound, a salt of radium and iodic acid with the chemical formula Ra(IO
3)
2.[1][2][3]
Synthesis
Radium iodate is obtained by the reaction of a soluble radium salt and iodic acid:[citation needed]
- RaCl
2 + 2HIO
3 → Ra(IO
3)
2 + 2HCl
- RaCl
Physical properties
Radium iodate forms colorless crystals. It is poorly soluble in water.[4]
References
- ↑ Haynes, William M. (22 June 2012) (in en). CRC Handbook of Chemistry and Physics, 93rd Edition. CRC Press. p. 5-197. ISBN 978-1-4398-8049-4. https://books.google.com/books?id=-BzP7Rkl7WkC&dq=radium+iodate&pg=PA51. Retrieved 14 June 2023.
- ↑ MaHam, Aihui; Ham, Bryan M. (1 October 2015) (in en). Analytical Chemistry: A Chemist and Laboratory Technician's Toolkit. John Wiley & Sons. p. 166. ISBN 978-1-119-06969-0. https://books.google.com/books?id=vFa-CgAAQBAJ&dq=radium+iodate&pg=PA166. Retrieved 14 June 2023.
- ↑ Macintyre, Jane E. (5 December 1996) (in en). Dictionary of Inorganic Compounds, Supplement 4. CRC Press. p. 484. ISBN 978-0-412-75020-5. https://books.google.com/books?id=VvDoE5oN09wC&dq=radium+iodate&pg=PA484. Retrieved 14 June 2023.
- ↑ Brown, Paul L.; Matyskin, Artem V.; Ekberg, Christian (1 June 2022). "The aqueous chemistry of radium" (in en). Radiochimica Acta 110 (6–9): 505–513. doi:10.1515/ract-2021-1141. ISSN 2193-3405.
 |

