Chemistry:Iodic acid
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Iodic(V) acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| HIO 3 | |||
| Molar mass | 175.91 g/mol | ||
| Appearance | White solid | ||
| Density | 4.62 g/cm3, solid | ||
| Melting point | 110 °C (230 °F; 383 K) | ||
| 269 g/100 mL (20 °C) | |||
| Acidity (pKa) | 0.75[1] | ||
| Conjugate base | Iodate | ||
| −48.0·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | acid, corrosive, oxidant | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other cations
|
Lithium iodate Potassium iodate | ||
Related halogen oxoacids
|
Chloric acid Bromic acid | ||
Related compounds
|
Hydroiodic acid Iodine pentoxide Periodic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
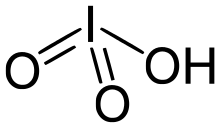
Iodic acid is a white water-soluble solid with the chemical formula HIO
3. Its robustness contrasts with the instability of chloric acid and bromic acid. Iodic acid features iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens. When heated, samples dehydrate to give iodine pentoxide. On further heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.
Preparation
Iodic acid can be produced by oxidizing iodine with strong oxidizers such as nitric acid, chlorine, chloric acid or hydrogen peroxide,[3] for example:
- I
2 + 6H
2O + 5Cl
2 ⇌ 2HIO
3 + 10HCl
Iodic acid is also produced by the reaction of iodine monochloride with water:
- 5ICl + 3H
2O → 5HCl + HIO
3 + 2I
2
Structure
Iodic acid crystallises from acidic solution as orthorhombic α-HIO3 in space group P212121. The structure consists of pyramidal molecules linked by hydrogen bonding and intermolecular iodine-oxygen interactions. The I=O bond lengths are 1.81 Å while the I–OH distance is 1.89 Å.[4][5][6] Several other polymorphs have been reported, including an orthorhombic γ form in space group Pbca[7] and an orthorhombic δ form in space group P212121.[8] All of the polymorphs contain pyramidal molecules, hydrogen bonding and I···O interactions, but differ in packing arrangement.
Properties
Iodic acid is a relatively strong acid with a pKa of 0.75. It is strongly oxidizing in acidic solution, less so in basic solution. When iodic acid acts as oxidizer, then the product of the reaction is either iodine, or iodide ion. Under some special conditions (very low pH and high concentration of chloride ions, such as in concentrated hydrochloric acid), iodic acid is reduced to iodine trichloride, a golden yellow compound in solution and no further reduction occurs. In the absence of chloride ions, when there is an excess amount of reductant, then all iodate is converted to iodide ion. When there is an excess amount of iodate, then part of the iodate is converted to iodine.[citation needed]
Uses
Iodic acid is used as a strong acid (though it is not truly a strong acid, but a weak acid that is very close to being a strong acid) in analytical chemistry. It may be used to standardize solutions of both weak and strong bases, using methyl red or methyl orange as the indicator.
Use in salt industry
Other oxyacids
Iodic acid is part of a series of oxyacids in which iodine can assume oxidation states of −1, +1, +3, +5, or +7. A number of neutral iodine oxides are also known.
| Iodine oxidation state | −1 | +1 | +3 | +5 | +7 |
|---|---|---|---|---|---|
| Name | Hydrogen iodide | Hypoiodous acid | Iodous acid | Iodic acid | Periodic acid |
| Formula | HI | HIO | HIO2 | HIO3 | HIO4 or H5IO6 |
References
- ↑ Perrin, D. D., ed (1982). Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). Entry 127. ISBN 0-08-029214-3.
- ↑ "Iodic acid" (in en). https://www.nwmissouri.edu/naturalsciences/sds/i/Iodic%20acid.pdf.
- ↑ Holleman, Arnold F.; Wiberg, Nils (2007) (in German). Lehrbuch der Anorganischen Chemie (102nd ed.). Berlin. ISBN 978-3-11-017770-1.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 863. ISBN 978-0-08-037941-8.
- ↑ Rogers, Max T.; Helmholz, Lindsay (1941). "The Crystal Structure of Iodic Acid". J. Am. Chem. Soc. 63 (1): 278–284. doi:10.1021/ja01846a068.
- ↑ Ståhl, Kenny; Szafranski, Marek (1992). "A Single-Crystal Neutron Diffraction Study of HIO3 at 295 and 30 K and of DIO3 at 295 K". Acta Chem. Scand. 46: 1146–1148. doi:10.3891/acta.chem.scand.46-1146.
- ↑ Fischer, Andreas; Lindsjö, Martin (2005). "γ-HIO3 – a Metastable, Centrosymmetric Polymorph of Iodic Acid". Z. Anorg. Allg. Chem. 631 (9): 1574–1576. doi:10.1002/zaac.200500099.
- ↑ Wu, Tao; Zavalij, Peter Y.; Zachariah, Michael R. (2017). "Crystal structure of a new polymorph of iodic acid, δ-HIO3, from powder diffraction". Powder Diffraction 32 (4): 261–264. doi:10.1017/S0885715617000859. Bibcode: 2017PDiff..32..261W.
 |