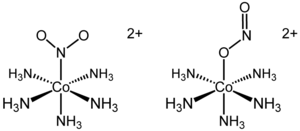Chemistry:Linkage isomerism
In chemistry, linkage isomerism or ambidentate isomerism is a form of isomerism in which certain coordination compounds have the same composition but differ in their metal atom's connectivity to a ligand.
Typical ligands that give rise to linkage isomers are:
- cyanide, CN−
– isocyanide, NC− - cyanate, OCN−
– isocyanate, NCO− - thiocyanate, SCN−
– isothiocyanate, NCS− - selenocyanate, SeCN−
– isoselenocyanate, NCSe− - nitrite, NO−
2 - sulfite, SO2−
3
Examples of linkage isomers are violet-colored [(NH
3)
5Co-SCN]2+ and orange-colored [(NH
3)
5Co-NCS]2+. The isomerization of the S-bonded isomer to the N-bonded isomer occurs intramolecularly.[1]
The complex cis-dichlorotetrakis(dimethylsulfoxide)ruthenium(II) (RuCl
2(dmso)
4) exhibits linkage isomerism of dimethyl sulfoxide ligands due to S- vs. O-bonding. Trans-dichlorotetrakis(dimethylsulfoxide)ruthenium(II) does not exhibit linkage isomers.
History
Linkage isomerism was first noted for nitropentaamminecobalt(III) chloride, [Co(NH
3)
5(NO
2)]2+. This cationic cobalt complex can be isolated as either of two linkage isomers. In the yellow-coloured isomer, the nitro ligand is bound through nitrogen. In the red linkage isomer, the nitrito is bound through one oxygen atom. The O-bonded isomer is often written as [Co(NH
3)
5(ONO)]2+. Although the existence of the isomers had been known since the late 1800s, only in 1907 was the difference explained.[2] It was later shown that the red isomer converted to the yellow isomer upon UV-irradiation. In this particular example, the formation of the nitro isomer (Co-NO
2) from the nitrito isomer (Co-ONO) occurs by an intramolecular rearrangement.[3]
References
- ↑ Buckingham, D. A.; Creaser, I. I.; Sargeson, A. M. (1970). "Mechanism of Base Hydrolysis for CoIII(NH3)5X2+ Ions. Hydrolysis and Rearrangement for the Sulfur-Bonded Co(NH3)5SCN2+ Ion". Inorg. Chem. 9 (3): 655–661. doi:10.1021/ic50085a044.
- ↑ Werner, A. (1907). "Über strukturisomere Salze der Rhodanwasserstoffsäure und der salpetrigen Säure" (in de). Ber. 40 (1): 765–788. doi:10.1002/cber.190704001117. https://zenodo.org/record/1426207.
- ↑ Basolo, Fred; Hammaker, G.S (1 February 1962). "Synthesis and Isomerization of Nitritopentammine Complexes of Rhodium(III), Iridium(III), and Platinum(IV)". Inorganic Chemistry 1 (1): 1–5. doi:10.1021/ic50001a001.
 |