Chemistry:Thiocyanate

| |
| Names | |
|---|---|
| Preferred IUPAC name
Thiocyanate[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| [SCN]− | |
| Molar mass | 58.08 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thiocyanates are salts containing the thiocyanate anion [SCN]−
(also known as rhodanide or rhodanate). [SCN]−
is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics.
Thiocyanate is analogous to the cyanate ion, [OCN]−
, wherein oxygen is replaced by sulfur. [SCN]−
is one of the pseudohalides, due to the similarity of its reactions to that of halide ions. Thiocyanate used to be known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron.
Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate with cyanide:
- [math]\ce{ 8 CN- + S8 -> 8 SCN- }[/math]
- [math]\ce{ CN- + S2O3^2- -> SCN- + SO3^2- }[/math]
The second reaction is catalyzed by thiosulfate sulfurtransferase, a hepatic mitochondrial enzyme, and by other sulfur transferases, which together are responsible for around 80% of cyanide metabolism in the body.[2]
Biological chemistry of thiocyanate in medicine
Thiocyanate[3] is known to be an important part in the biosynthesis of hypothiocyanite by a lactoperoxidase.[4][5][6] Thus the complete absence of thiocyanate or reduced thiocyanate[7] in the human body, (e.g., cystic fibrosis) is damaging to the human host defense system.[8][9]
Thiocyanate is a potent competitive inhibitor of the thyroid sodium-iodide symporter.[10] Iodine is an essential component of thyroxine. Since thiocyanates will decrease iodide transport into the thyroid follicular cell, they will decrease the amount of thyroxine produced by the thyroid gland. As such, foodstuffs containing thiocyanate are best avoided by iodide deficient hypothyroid patients.[11]
In the early 20th century, thiocyanate was used in the treatment of hypertension, but it is no longer used because of associated toxicity.[12] Sodium nitroprusside, a metabolite of which is thiocyanate, is however still used for the treatment of a hypertensive emergency. Rhodanese catalyzes the reaction of sodium nitroprusside with thiosulfate to form the metabolite thiocyanate. Thiocyanate is also a metabolite of the detoxification of cyanides by Rhodanese.
Coordination chemistry
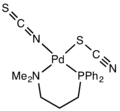
Thiocyanate shares its negative charge approximately equally between sulfur and nitrogen. As a consequence, thiocyanate can act as a nucleophile at either sulfur or nitrogen — it is an ambidentate ligand. [SCN]− can also bridge two (M−SCN−M) or even three metals (>SCN− or −SCN<). Experimental evidence leads to the general conclusion that class A metals (hard acids) tend to form N-bonded thiocyanate complexes, whereas class B metals (soft acids) tend to form S-bonded thiocyanate complexes. Other factors, e.g. kinetics and solubility, are sometimes involved, and linkage isomerism can occur, for example [Co(NH3)5(NCS)]Cl2 and [Co(NH3)5(SCN)]Cl2.[14] It [SCN] is considered as a weak ligand. ([NCS] is a strong ligand)[15]
Test for iron(III) and cobalt(II)
If [SCN]− is added to a solution with iron(III) ions, a blood-red solution forms mainly due to the formation of [Fe(SCN)(H2O)5]2+, i.e. pentaaqua(thiocyanato-N)iron(III). Lesser amounts of other hydrated compounds also form: e.g. Fe(SCN)3 and [Fe(SCN)4]−.[16]
Similarly, Co2+ gives a blue complex with thiocyanate.[17] Both the iron and cobalt complexes can be extracted into organic solvents like diethyl ether or amyl alcohol. This allows the determination of these ions even in strongly coloured solutions. The determination of Co(II) in the presence of Fe(III) is possible by adding KF to the solution, which forms uncoloured, very stable complexes with Fe(III), which no longer react with SCN−.[18]
Phospholipids or some detergents aid the transfer of thiocyanatoiron into chlorinated solvents like chloroform and can be determined in this fashion.[19]
See also
References
Citations
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. pp. 784, 1069. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Abraham, Klaus; Buhrke, Thorsten; Lampen, Alfonso (24 February 2015). "Bioavailability of cyanide after consumption of a single meal of foods containing high levels of cyanogenic glycosides: a crossover study in humans". Archives of Toxicology 90 (3): 559–574. doi:10.1007/s00204-015-1479-8. PMID 25708890.
- ↑ Pedemonte, N.; Caci, E.; Sondo, E.; Caputo, A.; Rhoden, K.; Pfeffer, U.; di Candia, M.; Bandettini, R. et al. (2007). "Thiocyanate Transport in Resting and IL-4-Stimulated Human Bronchial Epithelial Cells: Role of Pendrin and Anion Channels". Journal of Immunology 178 (8): 5144–5153. doi:10.4049/jimmunol.178.8.5144. PMID 17404297.
- ↑ Conner, G. E.; Wijkstrom-Frei, C.; Randell, S. H.; Fernandez, V. E.; Salathe, M. (2007). "The Lactoperoxidase System Links Anion Transport to Host Defense in Cystic Fibrosis". FEBS Letters 581 (2): 271–278. doi:10.1016/j.febslet.2006.12.025. PMID 17204267.
- ↑ White, W. E.; Pruitt, K. M.; Mansson-Rahemtulla, B. (1983). "Peroxidase-Thiocyanate-Peroxide Antibacterial System Does not Damage DNA". Antimicrobial Agents and Chemotherapy 23 (2): 267–272. doi:10.1128/aac.23.2.267. PMID 6340603.
- ↑ Thomas, E. L.; Aune, T. M. (1978). "Lactoperoxidase, Peroxide, Thiocyanate Antimicrobial System: Correlation of Sulfhydryl Oxidation with Antimicrobial Action". Infection and Immunity 20 (2): 456–463. doi:10.1128/IAI.20.2.456-463.1978. PMID 352945.
- ↑ Minarowski, Ł.; Sands, D.; Minarowska, A.; Karwowska, A.; Sulewska, A.; Gacko, M.; Chyczewska, E. (2008). "Thiocyanate concentration in saliva of cystic fibrosis patients". Folia Histochemica et Cytobiologica 46 (2): 245–246. doi:10.2478/v10042-008-0037-0. PMID 18519245. http://versita.metapress.com/content/12805r021413m867/fulltext.pdf.[no|permanent dead link|dead link}}]
- ↑ Moskwa, P.; Lorentzen, D.; Excoffon, K. J.; Zabner, J.; McCray, P. B. Jr.; Nauseef, W. M.; Dupuy, C.; Bánfi, B. (2007). "A Novel Host Defense System of Airways is Defective in Cystic Fibrosis". American Journal of Respiratory and Critical Care Medicine 175 (2): 174–183. doi:10.1164/rccm.200607-1029OC. PMID 17082494.
- ↑ Xu, Y.; Szép, S.; Lu, Z.; Szep; Lu (2009). "The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases". Proceedings of the National Academy of Sciences of the United States of America 106 (48): 20515–20519. doi:10.1073/pnas.0911412106. PMID 19918082. Bibcode: 2009PNAS..10620515X.
- ↑ Braverman L. E. et al. (2005). "The effect of perchlorate, thiocyanate, and nitrate on thyroid function in workers exposed to perchlorate long-term". J Clin Endocrinol Metab 90 (2): 700–706. doi:10.1210/jc.2004-1821. PMID 15572417. http://jcem.endojournals.org/content/90/2/700.long.
- ↑ "Hypothyroidism". University of Maryland Medical Center. http://umm.edu/health/medical/altmed/condition/hypothyroidism.
- ↑ Warren F. Gorman; Emanuel Messinger; And Morris Herman (1949). "Toxicity of Thiocyanates Used in Treatment of Hypertension". Ann Intern Med 30 (5): 1054–1059. doi:10.7326/0003-4819-30-5-1054. PMID 18126744.
- ↑ Palenik, Gus J.; Clark, George Raymond (1970). "Crystal and molecular structure of isothiocyanatothiocyanato-(1-diphenylphosphino-3-dimethylaminopropane)palladium(II)". Inorganic Chemistry 9 (12): 2754–2760. doi:10.1021/ic50094a028. ISSN 0020-1669.
- ↑ Greenwood, p. 326
- ↑ "coordination compounds". https://ncert.nic.in/ncerts/l/lech109.pdf.
- ↑ Greenwood, p. 1090
- ↑ Uri, N (1947-01-01). "The stability of the cobaltous thiocyanate complex in ethyl alcohol-water mixtures and the photometric determination of cobalt". Analyst 72 (860): 478–481. doi:10.1039/AN9477200478. PMID 18917685. Bibcode: 1947Ana....72..478U.
- ↑ Kolthoff, I. M. (1930). "The Cobalt-Thiocyanate Reaction for the Detection of Cobalt and Thiocyanate" (in en). Mikrochemie 8 (S1): 176–181. doi:10.1007/BF02759120. ISSN 0369-0261. http://link.springer.com/10.1007/BF02759120.
- ↑ Stewart, J.C. (1980). "Colorimetric determination of phospholipids with ammonium ferrothiocyanate". Anal. Biochem. 104 (1): 10–14. doi:10.1016/0003-2697(80)90269-9. PMID 6892980.
 |



