Chemistry:Dithiofluorescein
From HandWiki
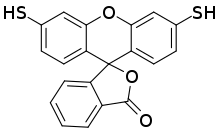
| |
| Names | |
|---|---|
| IUPAC name
3′,6′-Bis(sulfanyl)spiro[2-benzofuran-3,9′-xanthene]-1-one
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C20H12O3S2 | |
| Molar mass | 364.43 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dithiofluorescein (sometimes generically called thiofluorescein) is a complexometric indicator used in analytical chemistry. It changes from blue to colorless when it binds to mercury(2+) ions.[1] It thus can indicate the endpoint in the titration of thiols using o-hydroxymercuribenzoic acid[2] or its sodium salt.[3] The reagent can be immobilized t in a polymer on a fiber optic, which might allow development of a detector for sulfide ions in a flow cell.[4] Unlike fluorescein and other related fluoran dyes that have oxygen substituents on the benzene rings, dithiofluorescein, which has sulfur substituents, is not fluorescent.[5]
References
- ↑ Wroński, Mieczysław (1966). "Determination of thiol esters with o-hydroxymercuribenzoic acid". Analyst 19 (2): 745–746. doi:10.1039/AN9669100745.
- ↑ Wroński, Mieczysław (1977). "Analytical applications of o-hydroxymercuribenzoic acid, dithiofluorescein and mercurated fluorescein". Talanta 24 (6): 347–354. doi:10.1016/0039-9140(77)80019-2. PMID 18962100.
- ↑ Chromý, V.; Svoboda, V. (1963). "The determination of thiomalic acid". Talanta 10 (10): 1109–1111. doi:10.1016/0039-9140(63)80152-6.
- ↑ Narayanaswamy, Ramaier; Sevilla, Fortunato III (1986). "Flow cell studies with immobilised reagents for the development of an optical fibre sulphide sensor". Analyst 111 (9): 1085. doi:10.1039/AN9861101085.
- ↑ Sauer, Markus; Hofkens, Johan; Enderlein, Jörg (2011). "2.2. Organic Fluorophores". Handbook of Fluorescence Spectroscopy and Imaging: From Ensemble to Single Molecules. pp. 35–38. ISBN 978-3-527-31669-4.
 |

