Chemistry:DMDEE
| |
| Names | |
|---|---|
| IUPAC name
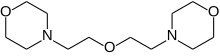
4-[2-(2-morpholin-4-ylethoxy)ethyl]morpholine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H24N2O3 | |
| Molar mass | 244.335 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319 | |
| P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P280, P302+352, P305+351+338, P321, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors | |
| Related compounds | |
Related compounds
|
1,2-Dimorpholinoethane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
DMDEE is an acronym for dimorpholinodiethyl ether but is almost always referred to as DMDEE (pronounced dumdee) in the polyurethane industry. It is an organic chemical, specifically a nitrogen-oxygen heterocycle with tertiary amine functionality. It is a catalyst used mainly to produce polyurethane foam. It has the CAS number 6425-39-4 and is TSCA and REACH registered and on EINECS with the number 229-194-7.[2] The IUPAC name is 4-[2-(2-morpholin-4-ylethoxy)ethyl]morpholine and the chemical formula C12H24N2O3.
Other names
Main section reference.[3]
- Morpholine, 4,4'-(oxydi-2,1-ethanediyl)bis-
- Bis(2-morpholinoethyl) Ether
- 4,4'-(Oxybis(ethane-2,1-diyl))dimorpholine
- 2,2-Dimorpholinodiethylether
- 2,2'-Dimorpholinodiethyl ether
- 4,4'-(Oxydiethylene)bis(morpholine)
- 4-[2-(2-morpholin-4-ylethoxy)ethyl]morpholine
- 2,2'-Dimorpholinyldiethyl ether
Use as a polyurethane catalyst
DMDEE tends to be used in one-component rather than 2-component polyurethane systems.[4][5] Its use has been investigated in polyurethanes for controlled drug release[6] and also adhesives for medical applications.[7] Its use as a catalyst including the kinetics[8] and thermodynamics have been studied and reported on extensively.[9][10][11][12][13] It is a popular catalyst along with DABCO.
Toxicity
The material has been in use for some time and so the toxicity is generally well understood.[14] However, some sources say toxicity data is limited and work continues to acquire the necessary data and publish to ensure it is in the public domain.[15][16]
References
- ↑ "Morpholine, 4,4'-(oxydi-2,1-ethanediyl)bis-" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/80900#section=Safety-and-Hazards.
- ↑ "DMDEE - morpholine" (in zh-CN). https://www.morpholine.org/dmdee/.
- ↑ "Morpholine, 4,4'-(oxydi-2,1-ethanediyl)bis-" (in en). PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/80900.
- ↑ "Amine Catalysis of Polyurethane Foams" (in en). Journal of Cellular Plastics 23 (5): 461–502. September 1987. doi:10.1177/0021955X8702300505. ISSN 0021-955X.
- ↑ "US Patent for Dimorpholinodiethylether having improved isocyanate stability Patent (Patent # 6,057,443 issued May 2, 2000) - Justia Patents Search". https://patents.justia.com/patent/6057443.
- ↑ "Catalyst-dependent drug loading of LDI-glycerol polyurethane foams leads to differing controlled release profiles". Acta Biomaterialia 4 (5): 1263–1274. September 2008. doi:10.1016/j.actbio.2008.01.008. PMID 18440884.
- ↑ "Development of a fast curing tissue adhesive for meniscus tear repair". Journal of Materials Science. Materials in Medicine 28 (1): 1. January 2017. doi:10.1007/s10856-016-5790-6. PMID 27866344.
- ↑ "A computational study on the mechanism and the kinetics of urethane formation" (in en). Computational and Theoretical Chemistry 963 (1): 168–175. January 2011. doi:10.1016/j.comptc.2010.10.017.
- ↑ "Urethane formation in the presence of 2,2-dimorpholinodiethylether (DMDEE) and 1,4-dimethylpiperazine (DMP) – A combined experimental and theoretical study" (in en). Computational and Theoretical Chemistry 1221: 114045. March 2023. doi:10.1016/j.comptc.2023.114045. ISSN 2210-271X.
- ↑ "Influence of Polar Modifiers on Microstructure of Polybutadiene Obtained by Anionic Polymerization. Part 2: Lewis Base (σ) Amine-Ether and Ether-Type Polar Modifiers" (in en). International Journal of Polymer Analysis and Characterization 20 (7): 602–611. 2015-10-03. doi:10.1080/1023666X.2015.1054079. ISSN 1023-666X.
- ↑ "Moisture curing kinetics of isocyanate ended urethane quasi-prepolymers monitored by IR spectroscopy and DSC" (in en). Journal of Applied Polymer Science 107 (2): 700–709. 2008-01-15. doi:10.1002/app.26453. https://onlinelibrary.wiley.com/doi/10.1002/app.26453.
- ↑ "Mechanism and kinetics of moisture-curing process of reactive hot melt polyurethane adhesive" (in en). Chemical Engineering Journal Advances 4: 100051. 2020-12-15. doi:10.1016/j.ceja.2020.100051. ISSN 2666-8211.
- ↑ "Recent Developments in Polyurethane Catalysis: Catalytic Mechanisms Review" (in en). Catalysis Reviews 46 (1): 31–51. 2004-12-26. doi:10.1081/CR-120027049. ISSN 0161-4940. http://www.tandfonline.com/doi/abs/10.1081/CR-120027049.
- ↑ "2,2'-Dimorpholinodiethyl ether - Hazardous Agents | Haz-Map". https://haz-map.com/Agents/10321.
- ↑ "Development of an Analytical Method for Quantitation of 2,2'-Dimorpholinodiethyl Ether (DMDEE) in Rat Plasma, Amniotic Fluid and Fetal Homogenate by UPLC-MS-MS for Determination of Gestational and Lactational Transfer in Rats". Journal of Analytical Toxicology 45 (9): 1036–1041. November 2021. doi:10.1093/jat/bkaa158. PMID 33031531.
- ↑ "Disposition and metabolism of 2,2'-dimorpholinodiethyl ether in sprague dawley rats and B6C3F1/N mice after oral, intravenous administration, and dermal application". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 50 (11): 1341–1351. November 2020. doi:10.1080/00498254.2020.1779389. PMID 32501166. https://figshare.com/articles/journal_contribution/12601396.
 |

