Chemistry:Ethyl caffeate
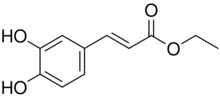 Chemical structure of ethyl caffeate
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl (2E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | |
| Other names
Caffeic acid ethyl ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H12O4 | |
| Molar mass | 208.213 g·mol−1 |
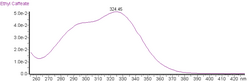
| UV-vis (λmax) | 324 nm and a shoulder at c. 295 nm in acidified methanol |
| Related compounds | |
Related compounds
|
Caffeic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethyl caffeate is an ester of a hydroxycinnamic acid, a naturally occurring organic compound.
Natural occurrences
It can be found in Bidens pilosa,[1] in Polygonum amplexicaule var. sinense.
It is also found in Huáng bǎi, one of the fifty fundamental herbs of traditional Chinese medicine, also known also as Cortex Phellodendri, the bark of one of two species of Phellodendron tree: Phellodendron amurense or Phellodendron chinense.[2]
It is also found in wines such as Verdicchio, a white wine from Marche, Italy.[3]
Health effects
Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2 and PGE2 in vitro or in mouse skin.[1]
Ethyl caffeate administered intraperitoneally in rats previously is able to prevent the dimethylnitrosamine-induced loss in body and liver weight, as well as to reduce the degree of liver injury. It can be considered as a promising natural compound for future application in chronic liver disease.[3]
Pharmacophore modeling, molecular docking, and molecular dynamics simulation studies also indicate that ethyl caffeate is a potential inhibitor of the aldosterone synthase (CYP11B2), a key enzyme for the biosynthesis of aldosterone, which plays a significant role for the regulation of blood pressure.[4]
Chemistry
Ethyl caffeate reacts with methylamine to produce green pigments.[5]
See also
- Phenolic compounds in wine
References
- ↑ 1.0 1.1 Chiang, Yi-Ming; Lo, Chiu-Ping; Chen, Yi-Ping; Wang, Sheng-Yang; Yang, Ning-Sun; Kuo, Yueh-Hsiung; Shyur, Lie-Fen (2005). "Ethyl caffeate suppresses NF-κB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2in vitroor in mouse skin". British Journal of Pharmacology 146 (3): 352–63. doi:10.1038/sj.bjp.0706343. PMID 16041399.
- ↑ Wang, M; Ji, TF; Yang, JB; Su, YL (2009). "Studies on the chemical constituents of Phellodendron chinense". Zhong Yao Cai 32 (2): 208–10. PMID 19504962.
- ↑ 3.0 3.1 Boselli, Emanuele; Bendia, Emanuele; Di Lecce, Giuseppe; Benedetti, Antonio; Frega, Natale G. (2009). "Ethyl caffeate from Verdicchio wine: Chromatographic purification and in vivo evaluation of its antifibrotic activity". Journal of Separation Science 32 (21): 3585–90. doi:10.1002/jssc.200900304. PMID 19813225.
- ↑ Luo, Ganggang; Lu, Fang; Qiao, Liansheng; Chen, Xi; Li, Gongyu; Zhang, Yanling (2016). "Discovery of Potential Inhibitors of Aldosterone Synthase from Chinese Herbs Using Pharmacophore Modeling, Molecular Docking, and Molecular Dynamics Simulation Studies". BioMed Research International 2016: 1–8. doi:10.1155/2016/4182595. ISSN 2314-6133. PMID 27781210.
- ↑ Matsui, T (1981). "Greening pigments produced reaction of ethyl caffeate with methylamine". Journal of Nutritional Science and Vitaminology 27 (6): 573–82. doi:10.3177/jnsv.27.573. PMID 7334427.
 |