Chemistry:Vinylferrocene
From HandWiki
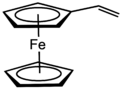
| |
| Names | |
|---|---|
| IUPAC name
Vinylferrocene
| |
| Systematic IUPAC name
Ethenylferrocene | |
| Other names
Ferrocenylethene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C12H12Fe | |
| Molar mass | 212.073 g·mol−1 |
| Appearance | orange solid |
| Melting point | 50–52 °C (122–126 °F; 323–325 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Vinylferrocene is the organometallic compound with the formula (C5H5)Fe(C5H4CH=CH2). It is a derivative of ferrocene, with a vinyl group attached to one cyclopentadienyl ligand. As the ferrocene analogue of styrene, it is the precursor to some polyferrocenes.[1] It is an orange, air-stable oily solid that is soluble in nonpolar organic solvents.
Vinylferrocene can be prepared by the dehydration of α-hydroxylethylferrocene, which is obtained from acetylferrocene.[2]
References
- ↑ Cass, Anthony E. G.; Davis, Graham; Francis, Graeme D.; Hill, H. Allen O.; Aston, William J.; Higgins, I. John; Plotkin, Elliot V.; Scott, Lesley D. L. et al. (1984). "Ferrocene-mediated enzyme electrode for amperometric determination of glucose". Analytical Chemistry 56 (4): 667–671. doi:10.1021/ac00268a018. PMID 6721151.
- ↑ Rausch, Marvin D.; Siegel, Armand (1968). "Organometallic π-complexes. XIV. Vinylmetallocenes". Journal of Organometallic Chemistry 11: 317–324. doi:10.1016/0022-328X(68)80054-3.
 |

