Chemistry:Rovafovir etalafenamide
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
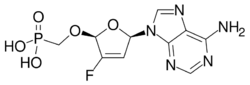
| Formula | C21H24FN6O6P |
| Molar mass | 506.431 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rovafovir etalafenamide (development code GS-9131) is an experimental drug for the treatment of HIV-1 infection.[1] Rovafovir etalafenamide is a nucleotide reverse transcriptase inhibitor and prodrug of GS-9148. Rovafovir etalafenamide itself has no antiviral activity, but once consumed it is metabolized through the hydrolysis of the phosphonoamidate group to generate the antiviral compound GS-9148.[1]
The drug is being developed by Gilead Sciences.[2]
Rovafovir etalafenamide shows antiviral activity against viruses containing major mutations associated with resistance to the nucleoside analog reverse-transcriptase inhibitors which are commonly used to treat HIV/AIDS infection.[1]
The methods by which the drug is synthesized by been published.[3][4][5]
References
- ↑ 1.0 1.1 1.2 "Rovafovir etalafenamide. Nucleotide reverse transcriptase inhibitor, Treatment of HIV-1 infection". Drugs of the Future 45 (7): 459. 2020. doi:10.1358/DOF.2020.45.7.3123468.
- ↑ "Rovafovir etalafenamide - Gilead Sciences". Adis Insight. https://adisinsight.springer.com/drugs/800016343.
- ↑ "Synthesis of Rovafovir Etalafenamide (Part I): Active Pharmaceutical Ingredient Process Development, Scale-Up, and Impurity Control Strategy". Organic Process Research & Development 25 (5): 1215–1236. 2021. doi:10.1021/acs.oprd.1c00059.
- ↑ "Synthesis of Rovafovir Etalafenamide (Part II): Dynamic Control for Successful Scale-Up of an Oxygen-Releasing Elimination Reaction Mediated by Oxone". Organic Process Research & Development 25 (5): 1237–1246. 2021. doi:10.1021/acs.oprd.0c00439.
- ↑ "Synthesis of Rovafovir Etalafenamide (Part III): Evolution of the Synthetic Process to the Phosphonamidate Fragment". Organic Process Research & Development 25 (5): 1247–1262. 2021. doi:10.1021/acs.oprd.0c00428.
 |