Chemistry:Monlunabant
From HandWiki
Short description: Chemical compound
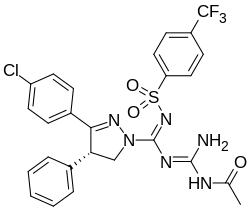 | |
| Clinical data | |
|---|---|
| Other names | INV-202, MRI-1891 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C26H22ClF3N6O3S |
| Molar mass | 591.01 g·mol−1 |
Monlunabant (INV-202, MRI-1891, or S-MRI-1891) is a peripherally selective cannabinoid receptor 1 inverse agonist, discovered as a β-arrestin-2-biased cannabinoid receptor 1 antagonist by the National Institutes of Health.[1] It was developed as a weight loss drug by Inversago Pharma.[2][3][4]
References
- ↑ Liu, Ziyi; Iyer, Malliga R.; Godlewski, Grzegorz; Jourdan, Tony; Liu, Jie; Coffey, Nathan J.; Zawatsky, Charles N.; Puhl, Henry L. et al. (11 June 2021). "Functional Selectivity of a Biased Cannabinoid-1 Receptor (CB 1 R) Antagonist". ACS Pharmacology & Translational Science 4 (3): 1175–1187. doi:10.1021/acsptsci.1c00048.</
- ↑ Crater, Glenn D.; Ravenelle, Francois; Lalonde, Karine; DespréS, Jean-Pierre (20 June 2023). "431-P: Effects of CB1 Antagonist INV-202 in Patients with Metabolic Syndrome—A Randomized, Placebo-Controlled, Double-Blind Phase 1B Study". Diabetes 72 (Supplement_1). doi:10.2337/db23-431-P.
- ↑ Morris, C.R.; Chandrasekaran, R.; Butzirius, I.; Daphtary, N.; Aliyeva, M.; Bates, J.H.T.; Anathy, V.; Crater, G.D. et al. (May 2023). "Cannabinoid Receptor 1 Inverse Agonist, INV-202, Induces Weight Loss and Reduces Airway Hyperreactivity in a Mouse Model of Obese Asthma". B15. Asthma: Hot off the Press from the Bench to the Clinic. pp. A2759. doi:10.1164/ajrccm-conference.2023.207.1_MeetingAbstracts.A2759.
- ↑ Crater, Glenn D.; Lalonde, Karine; Ravenelle, François; Harvey, Michael; Després, Jean-Pierre (8 November 2023). "Effects of CB1R inverse agonist, INV -202, in patients with features of metabolic syndrome. A randomized, placebo-controlled, double-blind phase 1b study". Diabetes, Obesity and Metabolism. doi:10.1111/dom.15353. PMID 37941317.
 |

