Chemistry:Panicudine
| |
| Names | |
|---|---|
| Other names
6-Hydroxy-11-deoxy-13-dehydrohetisane; (2α)-2,6-Dihydroxyhetisan-13-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C20H25NO3 | |
| Molar mass | 327.424 g·mol−1 |
| Melting point | 249–250 °C (480–482 °F; 522–523 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
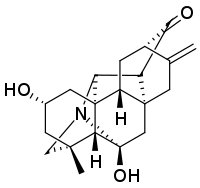
Panicudine (6-hydroxy-11-deoxy-13-dehydrohetisane) is a C20-diterpene alkaloid of the hetisine type, first isolated from Aconitum paniculatum. It has empirical formula C20H25NO3 and a melting point of 249–250 °C. The structure was determined to be a hetisine type diterpene by noting infrared spectrum absorption bands of 3405 cm−1 (OH), 1718 (C=O), and 1650 (C=C), a proton magnetic resonance spectrum with "secondary hydroxy (4.02 ppm, m, 1H, W1/2 = 10 Hz), exomethylene (4.87 and 4.76 ppm, br.s, 1H each), and tertiary methyl (1.29 ppm, s, 3H) groups and the absence of N-methyl, N-ethyl, and methoxy groups." Additional ultraviolet spectrum and carbon-13 NMR data, confirmed by high resolution mass spectrometry, completed the determination of the structure.[1]
Panicudine was identified as an active antimicrobial substance in the chloroform extract of Polygonum aviculare, a traditional herbal medicine of the Mediterranean coastal region.[2] It has also been isolated from epigeal parts of Rumex pictus.[3]
Related compounds
Panicutine is the acetate ester of panicudine.
A variety of related alkaloids have been isolated from other natural sources.[4][5]
References
- ↑ I. A. Bessonova, Sh. A. Saidkhodzhaeva and M. F. Faskhutdinov (1995). "Panicudine — A new alkaloid from Aconitum paniculatum". Chemistry of Natural Compounds 31 (6): 705–707. doi:10.1007/BF01386184.
- ↑ Hediat M.H. Salama; Najat Marraiki (January 2010). "Antimicrobial activity and phytochemical analyses of Polygonum aviculare L. (Polygonaceae), naturally growing in Egypt". Saudi Journal of Biological Sciences 17 (1): 57–63. doi:10.1016/j.sjbs.2009.12.009. PMID 23961059.
- ↑ Feng-Peng Wang (2002). C20-diterpenoid alkaloids. 59. 1–280. doi:10.1016/S0099-9598(02)59008-8. ISBN 9780124695597.
- ↑ F. N. Dzhakhangirov et al. (2007). "Toxicity and local anesthetic activity of diterpenoid alkaloids". Chemistry of Natural Compounds 43 (5): 581–589. doi:10.1007/s10600-007-0197-8.
- ↑ Phurpa Wangchuk, John B. Bremner and Siritron Samosorn (2007). "Hetisine-Type Diterpenoid Alkaloids from the Bhutanese Medicinal Plant Aconitum orochryseum". J. Nat. Prod. 70 (11): 1808–1811. doi:10.1021/np070266k. PMID 17966986.
 |


