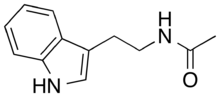
Chemistry:N-Acetyltryptamine
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
N-[2-(1H-indol-3-yl)ethyl]acetamide[1]
| |
| Other names
Acetotryptamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C12H14N2O | |
| Molar mass | 202.257 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Acetyltryptamine is an organic compound with the molecular formula C12H14N2O. It is a partial agonist for the melatonin receptors.[2][3][4] N-Acetyltryptamine is produced by Streptomyces djakartensis and other Streptomyces and Fusarium species.[5][6]
References
- ↑ "N-Acetyltryptamine" (in en). pubchem.ncbi.nlm.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/N-Acetyltryptamine#section=3D-Conformer.
- ↑ O'Brien, Paul (2 December 2012) (in en). Pineal and Retinal Relationships. Elsevier. p. 159. ISBN 978-0-323-14985-3.
- ↑ (in en) Current Trends in Comparative Endocrinology: Proceedings of the Ninth International Symposium on Comparative Endocrinology, Hong Kong, 7-11 December 1981. Kent State University Press. ISBN 978-962-209-116-0.
- ↑ "N-Acetyltryptamine (N10-Acetyltryptamine) | Melatonin Receptor Agonist | MedChemExpress". MedchemExpress.com. https://www.medchemexpress.com/n-acetyltryptamine.html.
- ↑ Zhang, Wenjuan; Wei, Shaopeng; Zhang, Jiwen; Wu, Wenjun (1 March 2013). "Antibacterial Activity Composition of the Fermentation Broth of Streptomyces djakartensis NW35". Molecules 18 (3): 2763–2768. doi:10.3390/molecules18032763.
- ↑ Paley, Elena L. (8 October 2020) (in en). Protein Biosynthesis Interference in Disease. Academic Press. p. 94. ISBN 978-0-12-823486-0.
Further reading
- Houlihan, William J. (15 September 2009) (in en). Indoles, Volume 25, Part 2. John Wiley & Sons. p. 25. ISBN 978-0-470-18841-5.
 |

