Chemistry:Nonafluoro-tert-butyl alcohol
| |
| Names | |
|---|---|
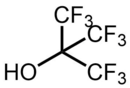
| Preferred IUPAC name
1,1,1,3,3,3-Hexafluoro-2-(trifluoromethyl)propan-2-ol | |
| Other names
perfluoro-tert-butyl alcohol, perfluoro-tert-butanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Hazards | |
| Main hazards | Corrosive, eye irritant |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nonafluoro-tert-butyl alcohol (IUPAC name: 1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol) is a fluoroalcohol. It is the perfluorinated analog of tert-butyl alcohol. Notably, as a consequence of its electron withdrawing fluorine substituents, it is very acidic for an alcohol, with a pKa value of 5.4, similar to that of a carboxylic acid. As another consequence of being a perfluorinated compound, it is also one of the lowest boiling alcohols, with a boiling point lower than that of methanol.
Synthesis
It is prepared by addition of trichloromethyllithium to hexafluoroacetone, followed by halogen exchange with antimony pentafluoride.[1] The aluminate derived from its alkoxide anion, tetrakis[1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-oxy]aluminate(1–), {Al[(CF3)3CO]4}– is used as a weakly coordinating anion.[2]
See also
- 2,2,2-Trifluoroethanol
- 1,1,1,3,3,3-Hexafluoro-2-propanol
- Hexafluoroacetone
- Perfluorotriethylcarbinol
References
- ↑ Filler, Robert; Schure, Ralph M. (1967-04-01). "Highly acidic perhalogenated alcohols. A new synthesis of perfluoro-tert-butyl alcohol" (in EN). The Journal of Organic Chemistry 32 (4): 1217–1219. doi:10.1021/jo01279a081. ISSN 0022-3263.
- ↑ Krossing, Ingo; Raabe, Ines (2004-04-13). "Noncoordinating Anions—Fact or Fiction? A Survey of Likely Candidates" (in en). Angewandte Chemie International Edition 43 (16): 2066–2090. doi:10.1002/anie.200300620. ISSN 1433-7851. PMID 15083452.
 |

