Chemistry:Ergosterol peroxide
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
(3S,5S,8S,9R,10R,13R,14R,17R)-10,13-dimethyl-17-[(1R,2E,4R)-1,4,5-trimethylhex-2-en-1-yl]-1,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-2H-5,8-epidioxycyclopenta[a]phenanthren-3-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H44O3 | |
| Molar mass | 428.647 |
| Density | 1.08g/cm3 |
| Boiling point | 499.7 °C (931.5 °F; 772.8 K) at 760mmHg |
| Hazards | |
| Flash point | 256 °C (493 °F; 529 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
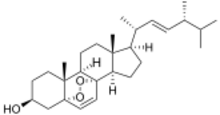
Ergosterol peroxide (5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol) is a steroid derivative. It has been isolated from a variety of fungi, yeast, lichens and sponges,[1][2][3][4][5] and has been reported to exhibit immunosuppressive,[6] anti-inflammatory,[7] antiviral,[8] trypanocidal [9] and antitumor activities in vitro.[10][11]
References
- ↑ "Triterpenoids of the fungus Pisolithus tinctorius". Phytochemistry 27 (11): 3569–74. 1988. doi:10.1016/0031-9422(88)80770-2. Bibcode: 1988PChem..27.3569L.
- ↑ "The dietary origin of epidioxy steroids in Actinia equina. A carbon14 incorporation experiment". Journal of Natural Products 52 (3): 619–22. 1989. doi:10.1021/np50063a023.
- ↑ "Ergosterol peroxide, an active compound from Inonotus radiatus". Planta Medica 55 (4): 389–90. 1989. doi:10.1055/s-2006-962036. PMID 2813575.
- ↑ "An ergostane derivative from the bark of Entandrophragma utile". Phytochemistry 31 (2): 704–705. 1992. doi:10.1016/0031-9422(92)90067-Z. Bibcode: 1992PChem..31..704T.
- ↑ "Isolation and characterization of immunosuppressive components of three mushrooms, Pisolithus tinctorius, Microporus flabelliformis and Lenzites betulina". Chem. Pharm. Bull. 42 (3): 694–97. 1994. doi:10.1248/cpb.42.694. PMID 8004718.
- ↑ "Inhibitory effects of sterols isolated from Chlorella vulgaris on 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and tumor promotion in mouse skin". Biological and Pharmaceutical Bulletin 19 (4): 573–576. 1996. doi:10.1248/bpb.19.573. PMID 8860961.
- ↑ "Antiviral activity of ergosterol peroxide". Pharmazie 44 (8): 579–80. 1989. PMID 2594833.
- ↑ "Screening of anti-HIV-1 activity of North American plants. Anti-HIV-1 activities of plant extracts, and active components of Lethalia vulpina (L.) Hue". Journal of Natural Medicines 52: 521–26. 1998.
- ↑ Ramos-Ligonio, Angel; López-Monteon, Aracely; Trigos, Ángel (2012-06-01). "Trypanocidal Activity of Ergosterol Peroxide from Pleurotus ostreatus" (in en). Phytotherapy Research 26 (6): 938–943. doi:10.1002/ptr.3653. ISSN 1099-1573. PMID 22083593.
- ↑ "Antitumor sterols from the mycelia of Cordyceps sinensis". Phytochemistry 51 (7): 891–98. 1999. doi:10.1016/S0031-9422(99)00128-4. PMID 10423860. Bibcode: 1999PChem..51..891B.
- ↑ "Cytotoxic activities of acetoxyscirpenediol and ergosterol peroxide from Paecilomyces tenuipes". Life Sciences 69 (2): 229–37. 2001. doi:10.1016/s0024-3205(01)01125-0. PMID 11441913.
 |

