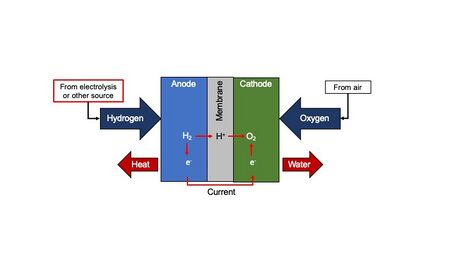
Chemistry:Electrocatalyst
An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode.[1] Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction.[2] Major challenges in electrocatalysts focus on fuel cells.[3][4]
Practical electrocatalysts
Chloralkali process
The chloralkali process is a large scale application that uses electrocatalysts. This technology supplies most of the chlorine and sodium hydroxide required by many industries. The cathode is a mixed metal oxide clad titanium anode (also called a dimensionally stable anode).[5][6]

Electrofluorination
Many organofluorine compounds are produced by electrofluorination.[7] One manifestation of this technology is the Simons process, which can be described as:
- R3C–H + HF → R3C–F + H2
In the course of a typical synthesis, this reaction occurs once for each C–H bond in the precursor. The cell potential is maintained near 5–6 V. The anode, the electrocatalyst, is nickel-plated.
Hydrodimerization of acrylonitrile
Acrylonitrile is converted to adiponitrile on an industrial scale via electrocatalysis.[1]
Background and theory
In general, a catalyst is an agent that increases the speed of a chemical reaction without being consumed by a reaction. Thermodynamically, a catalyst lowers the activation energy required for a chemical reaction to take place. An electrocatalyst is a catalyst that affects the activation energy of an electrochemical reaction.[8] Shown below is the activation energy of chemical reactions as it relates to the energies of products and reactants. The activation energy in electrochemical processes is related to the potential, i.e. voltage, at which a reaction occurs. Thus, electrocatalysts frequently change the potential at which oxidation and reduction processes are observed.[9] Alternatively, an electrocatalyst can be thought of as an agent that facilitates a specific chemical interaction at an electrode surface.[10] Given that electrochemical reactions occur when electrons are passed from one chemical species to another, favorable interactions at an electrode surface increase the likelihood of electrochemical transformations occurring, thus reducing the potential required to achieve these transformations.[10]

Electrocatalysts can be evaluated according to three figures of merit: activity, stability, and selectivity. The activity of electrocatalysts can be assessed quantitatively by understanding how much current density is generated, and therefore how fast a reaction is taking place, for a given applied potential. This relationship is described with the Tafel equation.[8] In assessing the stability of electrocatalysts, the ability of catalysts to withstand the potentials at which transformations are occurring is crucial. The selectivity of electrocatalysts refers to their preferential interaction with particular substrates, and their generation of a single product.[8] Selectivity can be quantitatively assessed through a selectivity coefficient, which compares the response of the material to the desired analyte or substrate with the response to other interferents.[11]
In many electrochemical systems, including galvanic cells, fuel cells and various forms of electrolytic cells, a drawback is that they can suffer from high activation barriers. The energy diverted to overcome these activation barriers is transformed into heat. In most exothermic combustion reactions this heat would simply propagate the reaction catalytically. In a redox reaction, this heat is a useless byproduct lost to the system. The extra energy required to overcome kinetic barriers is usually described in terms of low faradaic efficiency and high overpotentials.[8] In these systems, each of the two electrodes and its associated half-cell would require its own specialized electrocatalyst.[2]
Half-reactions involving multiple steps, multiple electron transfers, and the evolution or consumption of gases in their overall chemical transformations, will often have considerable kinetic barriers. Furthermore, there is often more than one possible reaction at the surface of an electrode. For example, during the electrolysis of water, the anode can oxidize water through a two electron process to hydrogen peroxide or a four electron process to oxygen. The presence of an electrocatalyst could facilitate either of the reaction pathways.[12]
Homogeneous electrocatalysts
A homogeneous electrocatalyst is one that is present in the same phase of matter as the reactants, for example, a water-soluble coordination complex catalyzing an electrochemical conversion in solution.[13][14] This technology is not practiced commercially, but is of research interest.
Synthetic coordination complexes
Many coordination complexes catalyze electrochemical reactions,[13][14] but only heterogeneous catalysts are of commercial value.
Enzymes
Some enzymes can function as electrocatalysts.[15] Nitrogenase, an enzyme that contains a MoFe cluster, can be leveraged to fix atmospheric nitrogen, i.e. convert nitrogen gas into molecules such as ammonia. Immobilizing the protein onto an electrode surface and employing an electron mediator greatly improves the efficiency of this process.[16] The effectiveness of bioelectrocatalysts generally depends on the ease of electron transport between the active site of the enzyme and the electrode surface.[15] Other enzymes provide insight for the development of synthetic catalysts. For example, formate dehydrogenase, a nickel-containing enzyme, has inspired the development of synthetic complexes with similar molecular structures for use in CO2 reduction.[17] Microbial fuel cells are another way that biological systems can be leveraged for electrocatalytic applications.[15][18] Microbial-based systems leverage the metabolic pathways of an entire organism, rather than the activity of a specific enzyme, meaning that they can catalyze a broad range of chemical reactions.[15] Microbial fuel cells can derive current from the oxidation of substrates such as glucose,[18] and be leveraged for processes such as CO2 reduction.[15]
Heterogeneous electrocatalysts
A heterogeneous electrocatalyst is one that is present in a different phase of matter from the reactants, for example, a solid surface catalyzing a reaction in solution. Different types of heterogeneous electrocatalyst materials are shown above in green. Since heterogeneous electrocatalytic reactions need an electron transfer between the solid catalyst (typically a metal) and the electrolyte, which can be a liquid solution but also a polymer or a ceramic capable of ionic conduction, the reaction kinetics depend on both the catalyst and the electrolyte as well as on the interface between them.[10] The nature of the electrocatalyst surface determines some properties of the reaction including rate and selectivity.[10]
Bulk materials
Electrocatalysis can occur at the surface of some bulk materials, such as platinum metal. Bulk metal surfaces of gold have been employed for the decomposition methanol for hydrogen production.[2] Water electrolysis is conventionally conducted at inert bulk metal electrodes such as platinum or iridium.[19] The activity of an electrocatalyst can be tuned with a chemical modification, commonly obtained by alloying two or more metals. This is due to a change in the electronic structure, especially in the d band which is considered to be responsible for the catalytic properties of noble metals.[20]
Nanomaterials
Nanoparticles
A variety of nanoparticle materials have been demonstrated to promote various electrochemical reactions,[21] although none have been commercialized. These catalysts can be tuned with respect to their size and shape, as well as the surface strain.[22]
Also, higher reaction rates can be achieved by precisely controlling the arrangement of surface atoms: indeed, in nanometric systems, the number of available reaction sites is a better parameter than the exposed surface area in order to estimate electrocatalytic activity. Sites are the positions where the reaction could take place; the likelihood of a reaction to occur in a certain site depends on the electronic structure of the catalyst, which determines the adsorption energy of the reactants together with many other variables not yet fully clarified.[23]
According to the TSK model, the catalyst surface atoms can be classified as terrace, step or kink atoms according to their position, each characterized by a different coordination number. In principle, atoms with lower coordination number (kinks and defects) tend to be more reactive and therefore adsorb the reactants more easily: this may promote kinetics but could also depress it if the adsorbing species isn't the reactant, thus inactivating the catalyst. Advances in nanotechnology make it possible to surface engineer the catalyst so that just some desired crystal planes are exposed to reactants, maximizing the number of effective reaction sites for the desired reaction.[21]
To date, a generalized surface dependence mechanism cannot be formulated since every surface effect is strongly reaction-specific. A few classifications of reactions based on their surface dependence have been proposed[23] but there are still many exceptions that do not fall into them.
Particle size effect
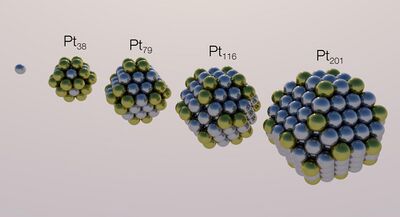
The interest in reducing as much as possible the costs of the catalyst for electrochemical processes led to the use of fine catalyst powders since the specific surface area increases as the average particle size decreases. For instance, most common PEM fuel cells and electrolyzers design is based on a polymeric membrane charged in platinum nanoparticles as an electrocatalyst (the so-called platinum black).[24]
Although the surface area to volume ratio is commonly considered to be the main parameter relating electrocatalyst size with its activity, to understand the particle-size effect, several more phenomena need to be taken into account:[23]
- Equilibrium shape: for any given size of a nanoparticle there is an equilibrium shape which exactly determines its crystal planes
- Reaction sites relative number: a given size for a nanoparticle corresponds to a certain number of surface atoms and only some of them host a reaction site
- Electronic structure: below a certain size, the work function of a nanoparticle changes and its band structure fades away
- Defects: the crystal lattice of a small nanoparticle is perfect; thus, reactions enhanced by defects as reaction sites get slowed down as the particle size decreases
- Stability: small nanoparticles have the tendency to lose mass due to the diffusion of their atoms towards bigger particles, according to the Ostwald ripening phenomenon
- Capping agents: in order to stabilize nanoparticles it is necessary a capping layer, therefore part of their surface is unavailable for reactants
- Support: nanoparticles are often fixed onto a support in order to stay in place, therefore part of their surface is unavailable for reactants
Carbon-based materials
Carbon nanotubes and graphene-based materials can be used as electrocatalysts.[25] The carbon surfaces of graphene and carbon nanotubes are well suited to the adsorption of many chemical species, which can promote certain electrocatalytic reactions.[26] In addition, their conductivity means they are good electrode materials.[26] Carbon nanotubes have a very high surface area, maximizing surface sites at which electrochemical transformations can occur.[27] Graphene can also serve as a platform for constructing composites with other kinds of nanomaterials such as single atom catalysts.[28] Because of their conductivity, carbon-based materials can potentially replace metal electrodes to perform metal-free electrocatalysis.[29]
Framework materials
Metal—organic frameworks (MOFs), especially conductive frameworks, can be used as electrocatalysts for processes such as CO2 reduction and water splitting. MOFs provide potential active sites at both metal centers and organic ligand sites.[30] They can also be functionalized, or encapsulate other materials such as nanoparticles.[30] MOFs can also be combined with carbon-based materials to form electrocatalysts.[31] Covalent organic frameworks (COFs), particularly those that contain metals, can also serve as electrocatalysts. COFs constructed from cobalt porphyrins demonstrated the ability to reduce carbon dioxide to carbon monoxide.[32]
However, many MOFs are known unstable in chemical and electrochemical conditions, making it difficult to tell if MOFs are actually catalysts or precatalysts. The real active sites of MOFs during electrocatalysis need to be analyzed comprehensively.[33]
Research on electrocatalysis
Water splitting / Hydrogen evolution
Hydrogen and oxygen can be combined through by the use of a fuel cell. In this process, the reaction is broken into two half reactions which occur at separate electrodes. In this situation the reactant's energy is directly converted to electricity. Useful energy can be obtained from the thermal heat of this reaction through an internal combustion engine with an upper efficiency of 60% (for compression ratio of 10 and specific heat ratio of 1.4) based on the Otto thermodynamic cycle. It is also possible to combine the hydrogen and oxygen through redox mechanism as in the case of a fuel cell. In this process, the reaction is broken into two half-reactions which occur at separate electrodes. In this situation the reactant's energy is directly converted to electricity.[34][35]
The standard reduction potential of hydrogen is defined as 0V, and frequently referred to as the standard hydrogen electrode (SHE).[36]
| Half Reaction | Reduction Potential
Eored (V) |
|---|---|
| 2H+ + 2e− → H2 (g) | ≡ 0 |
| O2(g) + 4H+ + 4e− → 2H2O(l) | +1.23 |
HER[13] can be promoted by many catalysts.[13]
Carbon dioxide reduction
Electrocatalysis for CO2 reduction is not practiced commercially but remains a topic of research. The reduction of CO2 into useable products is a potential way to combat climate change. Electrocatalysts can promote the reduction of carbon dioxide into methanol and other useful fuel and stock chemicals. The most valuable reduction products of CO2 are those that have a higher energy content, meaning that they can be reused as fuels. Thus, catalyst development focuses on the production of products such as methane and methanol.[14] Homogeneous catalysts, such as enzymes[17] and synthetic coordination complexes[14] have been employed for this purpose. A variety of nanomaterials have also been studied for CO2 reduction, including carbon-based materials and framework materials.[37]
Ethanol-powered fuel cells
Aqueous solutions of methanol can decompose into CO2 hydrogen gas, and water. Although this process is thermodynamically favored, the activation barrier is extremely high, so in practice this reaction is not typically observed. However, electrocatalysts can speed up this reaction greatly, making methanol a possible route to hydrogen storage for fuel cells.[2] Electrocatalysts such as gold, platinum, and various carbon-based materials have been shown to effectively catalyze this process. An electrocatalyst of platinum and rhodium on carbon backed tin-dioxide nanoparticles can break carbon bonds at room temperature with only carbon dioxide as a by-product, so that ethanol can be oxidized into the necessary hydrogen ions and electrons required to create electricity.[38]
Chemical synthesis
Electrocatalysts are used to promote certain chemical reactions to obtain synthetic products. Graphene and graphene oxides have shown promise as electrocatalytic materials for synthesis.[39] Electrocatalytic methods also have potential for polymer synthesis.[40] Electrocatalytic synthesis reactions can be performed under a constant current, constant potential, or constant cell-voltage conditions, depending on the scale and purpose of the reaction.[41]
Advanced oxidation processes in water treatment
Water treatment systems often require the degradation of hazardous compounds. These treatment processes are dubbed Advanced oxidation processes, and are key in destroying byproducts from disinfection, pesticides, and other hazardous compound. There is an emerging effort to enable these processes to destroy more tenacious compounds, especially PFAS[42]
Additional reading
- Valenti, G.; Boni, A.; Melchionna, M.; Cargnello, M.; Nasi, L.; Bertoli, G.; Gorte, R. J.; Marcaccio, M. et al. (2016). "Co-axial heterostructures integrating palladium/titanium dioxide with carbon nanotubes for efficient electrocatalytic hydrogen evolution". Nature Communications 7: 13549. doi:10.1038/ncomms13549. PMID 27941752. Bibcode: 2016NatCo...713549V.
See also
- Electrochemistry
- Catalysis
- Electrolysis of water
- Non-faradaic electrochemical modification of catalytic activity
- Tafel equation
References
- ↑ Jump up to: 1.0 1.1 Kotrel, Stefan; BrUninger, Sigmar (2008). "Industrial Electrocatalysis". Handbook of Heterogeneous Catalysis. doi:10.1002/9783527610044.hetcat0103. ISBN 978-3527312412.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 Roduner, Emil (June 13, 2017). "Selected fundamentals of catalysis and electrocatalysis in energy conversion reactions—A tutorial" (in en). Catalysis Today 309: 263–268. doi:10.1016/j.cattod.2017.05.091. https://linkinghub.elsevier.com/retrieve/pii/S0920586117304236.
- ↑ Debe, Mark K. (2012). "Electrocatalyst approaches and challenges for automotive fuel cells". Nature 486 (7401): 43–51. doi:10.1038/nature11115. PMID 22678278. Bibcode: 2012Natur.486...43D.
- ↑ Jiao, Yan; Zheng, Yao; Jaroniec, Mietek; Qiao, Shi Zhang (2015). "Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions". Chemical Society Reviews 44 (8): 2060–2086. doi:10.1039/C4CS00470A. PMID 25672249.
- ↑ Over, Herbert (2012). "Surface Chemistry of Ruthenium Dioxide in Heterogeneous Catalysis and Electrocatalysis: From Fundamental to Applied Research". Chemical Reviews 112 (6): 3356–3426. doi:10.1021/cr200247n. PMID 22423981.
- ↑ Landolt, D.; Ibl, N. (1972). "Anodic Chlorate Formation on Platinized Titanium". Journal of Applied Electrochemistry (Chapman and Hall Ltd.) 2 (3): 201–210. doi:10.1007/BF02354977.
- ↑ Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Jaramillo, Tom (September 3, 2014). "Electrocatalysis 101 | GCEP Symposium - October 11, 2012". https://www.youtube.com/watch?v=2sbsTLvcbCg&feature=youtu.be.
- ↑ Bard, Allen J.; Larry R. Faulkner (2001). Electrochemical methods: fundamentals and applications (Second ed.). Hoboken, NJ. ISBN 0-471-04372-9. OCLC 43859504.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 McCreery, Richard L. (July 2008). "Advanced Carbon Electrode Materials for Molecular Electrochemistry" (in en). Chemical Reviews 108 (7): 2646–2687. doi:10.1021/cr068076m. ISSN 0009-2665. PMID 18557655.
- ↑ Brown, Micah D.; Schoenfisch, Mark H. (2019-11-27). "Electrochemical Nitric Oxide Sensors: Principles of Design and Characterization" (in en). Chemical Reviews 119 (22): 11551–11575. doi:10.1021/acs.chemrev.8b00797. ISSN 0009-2665. PMID 31553169.
- ↑ Bard, Allen J.; Faulkner, Larry R. (January 2001). Electrochemical methods: fundamentals and applications. New York City: John Wiley & Sons. ISBN 978-0-471-04372-0. https://www.amazon.co.uk/gp/reader/0471043729/ref=sib_dp_pt#reader-link. Retrieved 27 February 2009.
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 Artero, Vincent; Chavarot-Kerlidou, Murielle; Fontecave, Marc (2011-08-01). "Splitting Water with Cobalt" (in en). Angewandte Chemie International Edition 50 (32): 7238–7266. doi:10.1002/anie.201007987. PMID 21748828. http://doi.wiley.com/10.1002/anie.201007987.
- ↑ Jump up to: 14.0 14.1 14.2 14.3 14.4 Kinzel, Niklas W.; Werlé, Christophe; Leitner, Walter (2021-01-19). "Transition Metal Complexes as Catalysts for the Electroconversion of CO 2 : An Organometallic Perspective" (in en). Angewandte Chemie International Edition 60 (21): 11628–11686. doi:10.1002/anie.202006988. ISSN 1433-7851. PMID 33464678.
- ↑ Jump up to: 15.0 15.1 15.2 15.3 15.4 Chen, Hui; Simoska, Olja; Lim, Koun; Grattieri, Matteo; Yuan, Mengwei; Dong, Fangyuan; Lee, Yoo Seok; Beaver, Kevin et al. (2020-12-09). "Fundamentals, Applications, and Future Directions of Bioelectrocatalysis" (in en). Chemical Reviews 120 (23): 12903–12993. doi:10.1021/acs.chemrev.0c00472. ISSN 0009-2665. PMID 33050699.
- ↑ Milton, Ross D.; Minteer, Shelley D. (2019-12-17). "Nitrogenase Bioelectrochemistry for Synthesis Applications" (in en). Accounts of Chemical Research 52 (12): 3351–3360. doi:10.1021/acs.accounts.9b00494. ISSN 0001-4842. PMID 31800207. https://pubs.acs.org/doi/abs/10.1021/acs.accounts.9b00494.
- ↑ Jump up to: 17.0 17.1 Yang, Jenny Y.; Kerr, Tyler A.; Wang, Xinran S.; Barlow, Jeffrey M. (2020-11-18). "Reducing CO 2 to HCO 2 – at Mild Potentials: Lessons from Formate Dehydrogenase" (in en). Journal of the American Chemical Society 142 (46): 19438–19445. doi:10.1021/jacs.0c07965. ISSN 0002-7863. PMID 33141560. https://pubs.acs.org/doi/10.1021/jacs.0c07965.
- ↑ Jump up to: 18.0 18.1 Qiao, Yan; Bao, Shu-Juan; Li, Chang Ming (2010). "Electrocatalysis in microbial fuel cells—from electrode material to direct electrochemistry" (in en). Energy & Environmental Science 3 (5): 544. doi:10.1039/b923503e. ISSN 1754-5692. http://xlink.rsc.org/?DOI=b923503e.
- ↑ Carmo, Marcelo; Fritz, David L.; Mergel, Jürgen; Stolten, Detlef (March 14, 2013). "A comprehensive review on PEM water electrolysis" (in en). International Journal of Hydrogen Energy 38 (12): 4901–4934. doi:10.1016/j.ijhydene.2013.01.151. https://linkinghub.elsevier.com/retrieve/pii/S0360319913002607.
- ↑ Mistry, H.; Varela, A.S.; Strasser, P.; Cuenya, B.R. (2016). "Nanostructured electrocatalysts with tunable activity and selectivity". Nature Reviews Materials 1 (4): 1–14. doi:10.1038/natrevmats.2016.9. Bibcode: 2016NatRM...116009M.
- ↑ Jump up to: 21.0 21.1 Kleijn, Steven E. F.; Lai, Stanley C. S.; Koper, Marc T. M.; Unwin, Patrick R. (2014-04-01). "Electrochemistry of Nanoparticles" (in en). Angewandte Chemie International Edition 53 (14): 3558–3586. doi:10.1002/anie.201306828. PMID 24574053. http://doi.wiley.com/10.1002/anie.201306828.
- ↑ Luo, Mingchuan; Guo, Shaojun (September 26, 2017). "Strain-controlled electrocatalysis on multimetallic nanomaterials" (in en). Nature Reviews Materials 2 (11): 17059. doi:10.1038/natrevmats.2017.59. ISSN 2058-8437. Bibcode: 2017NatRM...217059L. http://www.nature.com/articles/natrevmats201759.
- ↑ Jump up to: 23.0 23.1 23.2 Koper, M.T.M. (2011). "Structure sensitivity and nanoscale effects in electrocatalysis". Nanoscale (The Royal Society of Chemistry) 3 (5): 2054–2073. doi:10.1039/c0nr00857e. PMID 21399781. Bibcode: 2011Nanos...3.2054K.
- ↑ Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. (2013). "A comprehensive review on PEM water electrolysis". International Journal of Hydrogen Energy 38 (12): 4901–4934. doi:10.1016/j.ijhydene.2013.01.151.
- ↑ Wang, Xin (19 January 2008). "CNTs tuned to provide electrocatalyst support". Nanotechweb.org. http://nanotechweb.org/cws/article/tech/37366. Retrieved 27 February 2009.
- ↑ Jump up to: 26.0 26.1 McCreery, Richard L. (June 17, 2008). "Advanced Carbon Electrode Materials for Molecular Electrochemistry" (in en). Chemical Reviews 108 (7): 2646–2687. doi:10.1021/cr068076m. ISSN 0009-2665. PMID 18557655. https://pubs.acs.org/doi/10.1021/cr068076m.
- ↑ Wildgoose, Gregory G.; Banks, Craig E.; Leventis, Henry C.; Compton, Richard G. (November 30, 2005). "Chemically Modified Carbon Nanotubes for Use in Electroanalysis" (in en). Microchimica Acta 152 (3–4): 187–214. doi:10.1007/s00604-005-0449-x. ISSN 0026-3672. http://link.springer.com/10.1007/s00604-005-0449-x.
- ↑ Zhang, Qin; Zhang, Xiaoxiang; Wang, Junzhong; Wang, Congwei (2021-01-15). "Graphene-supported single-atom catalysts and applications in electrocatalysis". Nanotechnology 32 (3): 032001. doi:10.1088/1361-6528/abbd70. ISSN 0957-4484. PMID 33002887. Bibcode: 2021Nanot..32c2001Z. https://iopscience.iop.org/article/10.1088/1361-6528/abbd70.
- ↑ Dai, Liming (June 13, 2017). "Carbon-based catalysts for metal-free electrocatalysis" (in en). Current Opinion in Electrochemistry 4 (1): 18–25. doi:10.1016/j.coelec.2017.06.004.
- ↑ Jump up to: 30.0 30.1 Jiao, Long; Wang, Yang; Jiang, Hai-Long; Xu, Qiang (November 27, 2017). "Metal-Organic Frameworks as Platforms for Catalytic Applications" (in en). Advanced Materials 30 (37): 1703663. doi:10.1002/adma.201703663. PMID 29178384. http://doi.wiley.com/10.1002/adma.201703663.
- ↑ Singh, Chanderpratap; Mukhopadhyay, Subhabrata; Hod, Idan (January 5, 2021). "Metal–organic framework derived nanomaterials for electrocatalysis: recent developments for CO2 and N2 reduction" (in en). Nano Convergence 8 (1): 1. doi:10.1186/s40580-020-00251-6. ISSN 2196-5404. PMID 33403521. Bibcode: 2021NanoC...8....1S.
- ↑ Sharma, Rakesh Kumar; Yadav, Priya; Yadav, Manavi; Gupta, Radhika; Rana, Pooja; Srivastava, Anju; Zbořil, Radek; Varma, Rajender S. et al. (2020). "Recent development of covalent organic frameworks (COFs): synthesis and catalytic (organic-electro-photo) applications" (in en). Materials Horizons 7 (2): 411–454. doi:10.1039/C9MH00856J. ISSN 2051-6347. http://xlink.rsc.org/?DOI=C9MH00856J.
- ↑ Zheng, Weiran; Liu, Mengjie; Lee, Lawrence Yoon Suk (3 January 2020). "Electrochemical Instability of Metal–Organic Frameworks: In Situ Spectroelectrochemical Investigation of the Real Active Sites". ACS Catalysis 10 (1): 81–92. doi:10.1021/acscatal.9b03790.
- ↑ Kunze, Julia; Ulrich Stimming (2009). "Electrochemical Versus Heat-Engine Energy Technology: A Tribute to Wilhelm Ostwald's Visionary Statements". Angewandte Chemie International Edition 48 (49): 9230–9237. doi:10.1002/anie.200903603. PMID 19894237.
- ↑ Haverkamp, Richard (3 June 2008). "What is an electrocatalyst?" (QuickTime video and transcript). Science learning New Zealand. http://www.sciencelearn.org.nz/contexts/nanoscience/sci_media/video/what_is_an_electrocatalyst. Retrieved 27 February 2009.
- ↑ Elgrishi, Noémie; Rountree, Kelley J.; McCarthy, Brian D.; Rountree, Eric S.; Eisenhart, Thomas T.; Dempsey, Jillian L. (2018-02-13). "A Practical Beginner's Guide to Cyclic Voltammetry" (in en). Journal of Chemical Education 95 (2): 197–206. doi:10.1021/acs.jchemed.7b00361. ISSN 0021-9584. Bibcode: 2018JChEd..95..197E.
- ↑ Pan, Fuping; Yang, Yang (2020). "Designing CO 2 reduction electrode materials by morphology and interface engineering" (in en). Energy & Environmental Science 13 (8): 2275–2309. doi:10.1039/D0EE00900H. ISSN 1754-5692. http://xlink.rsc.org/?DOI=D0EE00900H.
- ↑ Harris, Mark (26 January 2009). "Booze-powered cars coming soon". techradar.com. Archived from the original on 2 March 2009. https://web.archive.org/web/20090302025300/http://www.techradar.com/news/world-of-tech/booze-powered-cars-coming-soon-513666. Retrieved 27 February 2009.
- ↑ Sachdeva, Harshita (2020-09-30). "Recent advances in the catalytic applications of GO/rGO for green organic synthesis". Green Processing and Synthesis 9 (1): 515–537. doi:10.1515/gps-2020-0055. ISSN 2191-9550.
- ↑ Siu, Juno C.; Fu, Niankai; Lin, Song (2020-03-17). "Catalyzing Electrosynthesis: A Homogeneous Electrocatalytic Approach to Reaction Discovery" (in en). Accounts of Chemical Research 53 (3): 547–560. doi:10.1021/acs.accounts.9b00529. ISSN 0001-4842. PMID 32077681.
- ↑ Holade, Yaovi; Servat, Karine; Tingry, Sophie; Napporn, Teko W.; Remita, Hynd; Cornu, David; Kokoh, K. Boniface (2017-10-06). "Advances in Electrocatalysis for Energy Conversion and Synthesis of Organic Molecules" (in en). ChemPhysChem 18 (19): 2573–2605. doi:10.1002/cphc.201700447. ISSN 1439-4235. PMID 28732139.
- ↑ Ji, Yangyuan; Choi, Youn Jeong; Fang, Yuhang; Pham, Hoang Son; Nou, Alliyan Tan; Lee, Linda S.; Niu, Junfeng; Warsinger, David M. (2023-01-19). "Electric Field-Assisted Nanofiltration for PFOA Removal with Exceptional Flux, Selectivity, and Destruction". Environmental Science & Technology (American Chemical Society (ACS)). doi:10.1021/acs.est.2c04874. ISSN 0013-936X. PMID 36657468.
 |