Chemistry:3-Dehydroshikimic acid
From HandWiki
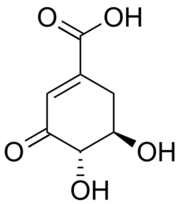
| |
| Names | |
|---|---|
| Preferred IUPAC name
(4S,5R)-4,5-Dihydroxy-3-oxocyclohex-1-ene-1-carboxylic acid | |
| Other names
3-Dehydroshikimate
3-DHS (−)-3-DHS | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C7H8O5 | |
| Molar mass | 172.136 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3-Dehydroshikimic acid is a chemical compound related to shikimic acid. 3-DHS is available in large quantity through engineering of the shikimic acid pathway.[1]
Metabolism
Biosynthesis: The enzyme 3-dehydroquinate dehydratase uses 3-dehydroquinate to produce 3-dehydroshikimate and H2O.
3-Dehydroshikimate is then reduced to shikimic acid by the enzyme shikimate dehydrogenase, which uses nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor.
Gallic acid is also formed from 3-dehydroshikimate by the action of the enzyme shikimate dehydrogenase to produce 3,5-didehydroshikimate. This latter compound spontaneously rearranges to gallic acid.[2][3][4]
References
- ↑ Banwell, M. G.; Edwards, A. J.; Essers, M.; Jolliffe, K. A. (2003). "Conversion of (−)-3-Dehydroshikimic Acid into Derivatives of the (+)-Enantiomer". The Journal of Organic Chemistry 68 (17): 6839–6841. doi:10.1021/jo034689c. PMID 12919063. https://figshare.com/articles/journal_contribution/3700032.
- ↑ Gallic acid pathway on metacyc.org
- ↑ Dewick, P. M.; Haslam, E. (1969). "Phenol biosynthesis in higher plants. Gallic acid". The Biochemical Journal 113 (3): 537–542. doi:10.1042/bj1130537. PMID 5807212.
- ↑ Muir, R. M.; Ibáñez, A. M.; Uratsu, S. L.; Ingham, E. S.; Leslie, C. A.; McGranahan, G. H.; Batra, N.; Goyal, S. et al. (2011). "Mechanism of gallic acid biosynthesis in bacteria (Escherichia coli) and walnut (Juglans regia)". Plant Molecular Biology 75 (6): 555–565. doi:10.1007/s11103-011-9739-3. PMID 21279669.
 |


