Biology:Lentiviral vector in gene therapy
Lentiviral vectors in gene therapy is a method by which genes can be inserted, modified, or deleted in organisms using lentiviruses. Lentiviruses are a family of viruses that are responsible for diseases like AIDS, which infect by inserting DNA into their host cells' genome.[1] Many such viruses have been the basis of research using viruses in gene therapy, but the lentivirus is unique in its ability to infect non-dividing cells, and therefore has a wider range of potential applications.[2] Lentiviruses can become endogenous (ERV), integrating their genome into the host germline genome, so that the virus is henceforth inherited by the host's descendants. Scientists use the lentivirus' mechanisms of infection to achieve a desired outcome to gene therapy. Lentiviral vectors in gene therapy have been pioneered by Luigi Naldini.[3][4]
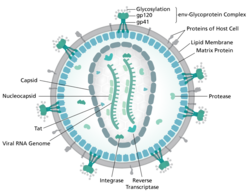
To understand the capabilities of a lentiviral vector, one has to consider the biology of the infection process.[editorializing] The lentivirus is a retrovirus, meaning it has a single stranded RNA genome with a reverse transcriptase enzyme. Lentiviruses also have a viral envelope with protruding glycoproteins that aid in attachment to the host cell's outer membrane. The virus contains a reverse transcriptase molecule found to perform transcription of the viral genetic material upon entering the cell. Within the viral genome are RNA sequences that code for specific proteins that facilitate the incorporation of the viral sequences into the host cell genome. The "gag" gene codes for the structural components of the viral nucleocapsid proteins: the matrix (MA/p17), the capsid (CA/p24) and the nucleocapsid (NC/p7) proteins. The "pol" domain codes for the reverse transcriptase and integrase enzymes. Lastly, the "env" domain of the viral genome encodes for the glycoproteins and envelope on the surface of the virus.[1][full citation needed]
There are multiple steps involved in the infection and replication of a lentivirus in a host cell. In the first step the virus uses its surface glycoproteins for attachment to the outer surface of a cell. More specifically, lentiviruses attach to the CD4 glycoproteins on the surface of a host's target cell. The viral material is then injected into the host cell's cytoplasm. Within the cytoplasm, the viral reverse transcriptase enzyme performs reverse transcription of the viral RNA genome to create a viral DNA genome. The viral DNA is then sent into the nucleus of the host cell where it is incorporated into the host cell's genome with the help of the viral enzyme integrase. From now on, the host cell starts to transcribe the entire viral RNA and express the structural viral proteins, in particular those that form the viral capsid and the envelope. The lentiviral RNA and the viral proteins then assemble and the newly formed virions leave the host cell when enough are made.[citation needed]
Two methods of gene therapy using lentiviruses have been proposed. In the ex vivo methodology, cells are extracted from a patient and then cultured. A lentiviral vector carrying therapeutic transgenes are then introduced to the culture to infect them. The now modified cells continue to be cultured until they can be infused into the patient. In vivo gene therapy is the sample injection of viral vectors containing transgenes into the patient.[5]
Designing a lentivirus vector
Lentiviruses are modified to act as a vector to insert beneficial genes into cells. Unlike other retroviruses, which cannot penetrate the nuclear envelope and can therefore only act on cells while they are undergoing mitosis, lentiviruses can infect cells whether or not they are dividing (shown to be largely due to the capsid protein).[6] Many cell types, like neurons, do not divide in adult organisms, so lentiviral gene therapy is a good candidate for treating conditions that affect those cell types.[7]
Some experimental applications of lentiviral vectors[8] have been done in gene therapy in order to cure diseases like Diabetes mellitus, Murine haemophilia A, prostate cancer, chronic granulomatous disease, and vascular diseases.[citation needed]
HIV-derived lentiviral vectors have been widely developed for their ability to target specific genes through the coactivator PSIP1.[9] This target specificity allows for the development of lentiviral gene vectors that do not carry the risk of randomly inserting themselves into normally functioning genes. As HIV is pathogenic, it must be genetically modified to remove its disease-causing properties and its ability to replicate itself. This can be achieved by deleting viral genes that are unnecessary for transduction of therapeutic transgenes. It has been proposed that by targeting the "gag" and "env" domains, enough of the HIV-1 genome can be deleted without losing its effectiveness in gene therapy while minimizing viral genes integrated into the patient.[10] Genes may also be replaced rather than disrupted as another method to reduce the risks associated with the use of HIV-1.[7]
Other lentiviruses such as Feline immunodeficiency virus[11] and Equine infectious anemia virus[12] have been developed for use in gene therapy and are of interest due to the inability to cause serious disease in human hosts. Equine infectious anemia virus in particular has been shown to perform somewhat better than HIV-1 in hematopoietic stem cells[13]
Insertional mutagenesis
Historically, lentiviral vectors included strong viral promoters which had a side effect of insertional mutagenesis, nuclear DNA mutations that affect the function of a gene.[14] These strong viral promotors were shown to be the main cause of cancer formation.[14] As a result, viral promotors have been replaced by cellular promotors and regulatory sequences.[14]
Contrast with other viral vectors
As mentioned, lentiviruses have the unique ability to infect non-dividing cells. Beyond that, there are several other properties that distinguish lentiviral vectors from other viral vectors. Such properties are important to consider when determining whether lentiviruses are appropriate for a given treatment.
Gammaretroviruses
Gammaretroviruses are retroviruses like lentiviruses. Murine leukemia viruses (MLVs) were among the first to be investigated for their use in gene therapy. However, recent research has favored lentiviruses for their ability to integrate into non-dividing cells. More practically, gammaretroviruses have an affinity for integrating themselves near oncogene promoters, bringing forward an adverse risk of tumors.[15] MLVs may be replication competent, meaning they can replicate in the host cell. These replication-competent viruses offer stable gene transfer and tumor and tissue specific targeting.[16]
Adenoviruses
In gene therapy, adenoviruses differ from lentiviruses in many ways, some of which provide advantages over lentiviruses. Transduction efficiency is higher in adenoviruses compared to lentiviruses.[5] Secondly, most human cells have receptors for adenoviruses[17] likely as a result of the wide variety of adenovirus diseases in humans. However, this presents a drawback[editorializing] - as adenoviruses frequently infect humans, this builds an immune response in the body. Such a response can reduce the efficiency of adenoviral vector therapies and can result in adverse reactions such as inflammation of tissues.[18] Research has been conducted to exploit this immune response to target cancerous cells and to develop vaccines.[19] Hybrid adenovirus-retroviruses (specifically MLVs) have also been developed to exploit the benefits of MLVs and adenoviruses.[20]
Applications
Severe combined immunodeficiency disease
The ADA deficient variant of severe combined immunodeficiency (SCID) was treated highly successfully in a multi-year study reported in 2021. Over 95% of treated patients continued to be event free after 36 months, and 100% of patients survived this normally lethal disease. A self-inactivating lentiviral vector, EFS-ADA LV, was used to insert a functional ADA gene in autologous CD34+ hematopoietic stem and progenitor cells (HSPCs).[21]
Vascular transplants
In a study designed to enhance the outcomes of vascular transplant through vascular endothelial cell gene therapy, the third generation of Lentivirus showed to be effective in the delivery of genes to moderate venous grafts and transplants in procedures like coronary artery bypass. Because the virus has been adapted to lose most of its genome, the virus becomes safer and more effective in transplanting the required genes into the host cell. A drawback to this therapy is explained in the study that long-term gene expression may require the use of promoters and can aid in a greater trans-gene expression. The researchers accomplished this by the addition of self-inactivating plasmids and creating a more universal tropism by pseudotyping a vesicular stomatitis virus glycoprotein.[22]
Chronic granulomatous disease
In chronic granulomatous disease (CGD), immune functioning is deficient as a result of the mutations in components of the nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) enzyme in phagocyte cells, which catalyzes the production of superoxide free radicals. If this enzyme becomes deficient, the phagocytes can't kill effectively the engulfed bacteria, so granulomas can be formed. Study performed in mice emphasizes the use of lineage-specific lentiviral vectors to express a normal version of one of the mutant CGD proteins, allowing white blood cells to now make a functional version of the NADPH oxidase. Scientists developed this strain of lentivirus by transinfecting 293T cells with pseudotyped virus with the vesicular stomatitis G protein. The viral vector's responsibility was to increase the production of a functional NADPH oxidase gene in these phagocytic cells. They did this to create an affinity for myeloid cells.[23]
Prostate cancer
With prostate cancer, the lentivirus is transformed by being bound to trastuzumab to attach to androgen-sensitive LNCaP and castration-resistant C4-2 human prostate cancer cell lines. These two cells are primarily responsible for secretion of excess human epidermal growth factor receptor 2 (HER-2), which is a hormone linked to prostate cancer. By attaching to these cells and changing their genomes, the lentivirus can slow down, and even kill, the cancer-causing cells. Researchers caused the specificity of the vector by manipulating the Fab region of the viral genome and pseudotyped it with the Sindbis virus.[24]
Haemophilia A
Haemophilia A has also been studied in gene therapy with a lentiviral vector in mice. The vector targets the haematopoietic cells in order to increase the amount of factor VIII, which is affected in haemophilia A. But this continues to be a subject of study as the lentivirus vector was not completely successful in achieving this goal. They did this by trans-infecting the virus in a 293T cell, creating a virus known as 2bF8 expressing generation of viral vectors.[25]
Rheumatoid arthritis
Studies have also found that injection of a lentiviral vector with IL-10 expressing genes in utero in mice can suppress, and prevent, rheumatoid arthritis and create new cells with constant gene expression. This contributes to the data on stem cells and in utero inoculation of viral vectors for gene therapy. The target for the viral vector in this study, were the synovial cells. Normally functioning synovial cells produce TNFα and IL-1.[26]
Diabetes mellitus
Like many of the in utero studies, the lentiviral vector gene therapy for diabetes mellitus is more effective in utero as the stem cells that become affected by the gene therapy create new cells with the new gene created by the actual viral intervention. The vector targets the cells within the pancreas to add insulin secreting genes to help control diabetes mellitus. Vectors were cloned using a cytomegalovirus promoter and then co-transinfected in the 293T cell.[27]
Neurological disease
As mature neurons do not divide, lentiviruses are ideal for division independent gene therapy. Studies of lentiviral gene therapy have been conducted on patients with advanced Parkinson's disease[28] and aging-related atrophy of neurons in primates.[29]
See also
References
- ↑ Milone, Michael C.; O’Doherty, Una (July 2018). "Clinical use of lentiviral vectors" (in en). Leukemia 32 (7): 1529–1541. doi:10.1038/s41375-018-0106-0. ISSN 1476-5551. PMC 6035154. https://www.nature.com/articles/s41375-018-0106-0.
- ↑ Cockrell, Adam S.; Kafri, Tal (2007-07-01). "Gene delivery by lentivirus vectors". Molecular Biotechnology 36 (3): 184–204. doi:10.1007/s12033-007-0010-8. ISSN 1073-6085. PMID 17873406.
- ↑ "Hear Luigi Naldini, MD, PhD, on the Giants of Gene Therapy Podcast | ASGCT - American Society of Gene & Cell Therapy". https://asgct.org/publications/luigi-naldini-md-phd-giants-of-gene-therapy-podcast.
- ↑ Naldini, Luigi; Blömer, Ulrike; Gallay, Philippe; Ory, Daniel; Mulligan, Richard; Gage, Fred H.; Verma, Inder M.; Trono, Didier (1996-04-12). "In Vivo Gene Delivery and Stable Transduction of Nondividing Cells by a Lentiviral Vector" (in en). Science 272 (5259): 263–267. doi:10.1126/science.272.5259.263. ISSN 0036-8075. https://www.science.org/doi/10.1126/science.272.5259.263.
- ↑ 5.0 5.1 Bulcha, Jote T.; Wang, Yi; Ma, Hong; Tai, Phillip W. L.; Gao, Guangping (December 2021). "Viral vector platforms within the gene therapy landscape" (in en). Signal Transduction and Targeted Therapy 6 (1): 53. doi:10.1038/s41392-021-00487-6. ISSN 2059-3635. PMID 33558455.
- ↑ "Capsid Is a Dominant Determinant of Retrovirus Infectivity in Nondividing Cells". Journal of Virology 78 (11): 5670–5678. June 2004. doi:10.1128/JVI.78.11.5670-5678.2004. PMID 15140964.
- ↑ 7.0 7.1 "Development of lentiviral vectors for gene therapy for human diseases". Blood 95 (8): 2499–504. April 2000. doi:10.1182/blood.V95.8.2499. PMID 10753827.
- ↑ "What are lentiviral vectors?". http://biology.kenyon.edu/slonc/gene-web/Lentiviral/Lentivi2.html.
- ↑ Gijsbers, Rik; Ronen, Keshet; Vets, Sofie; Malani, Nirav; De Rijck, Jan; McNeely, Melissa; Bushman, Frederic D; Debyser, Zeger (March 2010). "LEDGF Hybrids Efficiently Retarget Lentiviral Integration Into Heterochromatin" (in en). Molecular Therapy 18 (3): 552–560. doi:10.1038/mt.2010.36. PMID 20195265.
- ↑ Sertkaya, Helin; Ficarelli, Mattia; Sweeney, Nathan P.; Parker, Hannah; Vink, Conrad A.; Swanson, Chad M. (December 2021). "HIV-1 sequences in lentiviral vector genomes can be substantially reduced without compromising transduction efficiency" (in en). Scientific Reports 11 (1): 12067. doi:10.1038/s41598-021-91309-w. ISSN 2045-2322. PMID 34103612. Bibcode: 2021NatSR..1112067S.
- ↑ Djalilian, Hamid R; Tsuboi, Yasuhiro; Ozeki, Masashi; Tekin, Muhammet; Djalilian, Ali R; Obritch, Wesley; Lin, Jizhen (April 2002). "Feline immunodeficiency virus-mediated gene therapy of middle ear mucosa cells" (in en). Auris Nasus Larynx 29 (2): 183–186. doi:10.1016/S0385-8146(01)00131-6. PMID 11893454. https://linkinghub.elsevier.com/retrieve/pii/S0385814601001316.
- ↑ Binley, Katie; Widdowson, Peter S.; Kelleher, Michelle; de Belin, Jackie; Loader, Julie; Ferrige, Georgina; Carlucci, Marie; Esapa, Margaret et al. (September 2012). "Safety and Biodistribution of an Equine Infectious Anemia Virus-Based Gene Therapy, RetinoStat ® , for Age-Related Macular Degeneration" (in en). Human Gene Therapy 23 (9): 980–991. doi:10.1089/hum.2012.008. ISSN 1043-0342. PMID 22716662. http://www.liebertpub.com/doi/10.1089/hum.2012.008.
- ↑ O'Rourke, J. P.; Olsen, J. C.; Bunnell, B. A. (January 2005). "Optimization of equine infectious anemia derived vectors for hematopoietic cell lineage gene transfer" (in en). Gene Therapy 12 (1): 22–29. doi:10.1038/sj.gt.3302350. ISSN 1476-5462. PMID 15550928. https://www.nature.com/articles/3302350.
- ↑ 14.0 14.1 14.2 Poletti, Valentina; Mavilio, Fulvio (2021). "Designing Lentiviral Vectors for Gene Therapy of Genetic Diseases" (in en). Viruses 13 (8): 5. doi:10.3390/v13081526. ISSN 1999-4915. PMID 34452394.
- ↑ Shinkuma, Satoru (July 2021). "Advances in gene therapy and their application to skin diseases: A review" (in en). Journal of Dermatological Science 103 (1): 2–9. doi:10.1016/j.jdermsci.2021.05.004. PMID 34049771. https://linkinghub.elsevier.com/retrieve/pii/S0923181121001109.
- ↑ Metzl, Christian; Mischek, Daniela; Salmons, Brian; Günzburg, Walter H.; Renner, Matthias; Portsmouth, Daniel (2006-07-15). "Tissue- and Tumor-Specific Targeting of Murine Leukemia Virus-Based Replication-Competent Retroviral Vectors" (in en). Journal of Virology 80 (14): 7070–7078. doi:10.1128/JVI.00020-06. ISSN 0022-538X. PMID 16809312.
- ↑ Zhang, Yuanming; Bergelson, Jeffrey M. (October 2005). "Adenovirus Receptors" (in en). Journal of Virology 79 (19): 12125–12131. doi:10.1128/JVI.79.19.12125-12131.2005. ISSN 0022-538X. PMID 16160140.
- ↑ Appledorn, Daniel M.; Patial, Sonika; McBride, Aaron; Godbehere, Sarah; Van Rooijen, Nico; Parameswaran, Narayanan; Amalfitano, Andrea (2008-08-01). "Adenovirus Vector-Induced Innate Inflammatory Mediators, MAPK Signaling, As Well As Adaptive Immune Responses Are Dependent upon Both TLR2 and TLR9 In Vivo" (in en). The Journal of Immunology 181 (3): 2134–2144. doi:10.4049/jimmunol.181.3.2134. ISSN 0022-1767. PMID 18641352.
- ↑ Zhang, Chao; Zhou, Dongming (2016-08-02). "Adenoviral vector-based strategies against infectious disease and cancer" (in en). Human Vaccines & Immunotherapeutics 12 (8): 2064–2074. doi:10.1080/21645515.2016.1165908. ISSN 2164-5515. PMID 27105067.
- ↑ Kubo, Shuji; Haga, Kazunori; Tamamoto, Atsuko; Palmer, Donna J; Ng, Philip; Okamura, Haruki; Kasahara, Noriyuki (January 2011). "Adenovirus–Retrovirus Hybrid Vectors Achieve Highly Enhanced Tumor Transduction and Antitumor Efficacy In Vivo" (in en). Molecular Therapy 19 (1): 76–82. doi:10.1038/mt.2010.182. PMID 20808291.
- ↑ Kohn, DB; Booth, C; Shaw, KL; Xu-Bayford, J; Garabedian, E; Trevisan, V; Carbonaro-Sarracino, DA; Soni, K et al. (27 May 2021). "Autologous Ex Vivo Lentiviral Gene Therapy for Adenosine Deaminase Deficiency.". The New England Journal of Medicine 384 (21): 2002–2013. doi:10.1056/NEJMoa2027675. PMID 33974366.
- ↑ "Third-generation lentivirus vectors efficiently transduce and phenotypically modify vascular cells: implications for gene therapy". J. Mol. Cell. Cardiol. 35 (7): 739–48. July 2003. doi:10.1016/S0022-2828(03)00136-6. PMID 12818564.
- ↑ "Lineage- and stage-restricted lentiviral vectors for the gene therapy of chronic granulomatous disease". Gene Ther. 18 (11): 1087–97. November 2011. doi:10.1038/gt.2011.65. PMID 21544095.
- ↑ "Lentiviruses with trastuzumab bound to their envelopes can target and kill prostate cancer cells". Cancer Gene Ther. 16 (11): 820–31. November 2009. doi:10.1038/cgt.2009.28. PMID 19373278.
- ↑ "Lentivirus-mediated platelet-derived factor VIII gene therapy in murine haemophilia A". J. Thromb. Haemost. 5 (2): 352–61. February 2007. doi:10.1111/j.1538-7836.2007.02346.x. PMID 17269937.
- ↑ "Early gestational gene transfer of IL-10 by systemic administration of lentiviral vector can prevent arthritis in a murine model". Gene Ther. 18 (7): 719–26. July 2011. doi:10.1038/gt.2011.23. PMID 21390071.
- ↑ "Gene therapy for diabetes mellitus in rats by intramuscular injection of lentivirus containing insulin gene". Diabetes Res. Clin. Pract. 71 (3): 233–40. March 2006. doi:10.1016/j.diabres.2005.08.005. PMID 16171885.
- ↑ Palfi, Stéphane; Gurruchaga, Jean Marc; Ralph, G. Scott; Lepetit, Helene; Lavisse, Sonia; Buttery, Philip C.; Watts, Colin; Miskin, James et al. (2014-03-29). "Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, phase 1/2 trial" (in English). The Lancet 383 (9923): 1138–1146. doi:10.1016/S0140-6736(13)61939-X. ISSN 0140-6736. PMID 24412048. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)61939-X/abstract.
- ↑ Nagahara, Alan H.; Bernot, Tim; Moseanko, Rod; Brignolo, Laurie; Blesch, Armin; Conner, James M.; Ramirez, Anthony; Gasmi, Mehdi et al. (January 2009). "Long-term reversal of cholinergic neuronal decline in aged non-human primates by lentiviral NGF gene delivery" (in en). Experimental Neurology 215 (1): 153–159. doi:10.1016/j.expneurol.2008.10.004. PMID 19013154.
Further reading
- "Development of lentiviral vectors for gene therapy for human diseases". Blood 95 (8): 2499–504. April 2000. doi:10.1182/blood.V95.8.2499. PMID 10753827.
- "Lentiviral vectors in gene therapy: their current status and future potential". Arch. Immunol. Ther. Exp. (Warsz.) 58 (2): 107–19. April 2010. doi:10.1007/s00005-010-0063-4. PMID 20143172.
- "Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease". Nat. Med. 16 (2): 198–204. February 2010. doi:10.1038/nm.2088. PMID 20098431. http://publikationen.ub.uni-frankfurt.de/files/33767/nm.2088.pdf.
- Persons DA (May 2010). "Lentiviral vector gene therapy: effective and safe?". Mol. Ther. 18 (5): 861–2. doi:10.1038/mt.2010.70. PMID 20436489.
External links
- The Place of Retroviruses in Biology
- Synthesis of Gag and Gag-Pro-Pol Proteins in Retroviruses
- About: Retroviruses Resource Overview
 |