Biology:Genome
| Part of a series on |
| Genetics |
|---|
 |
| Key components |
| History and topics |
| Research |
| Personalized medicine |
| Personalized medicine |

In the fields of molecular biology and genetics, a genome is all the genetic information of an organism.[1] It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding genes, other functional regions of the genome such as regulatory sequences (see non-coding DNA), and often a substantial fraction of junk DNA with no evident function.[2][3] Almost all eukaryotes have mitochondria and a small mitochondrial genome.[2] Algae and plants also contain chloroplasts with a chloroplast genome.
The study of the genome is called genomics. The genomes of many organisms have been sequenced and various regions have been annotated. The Human Genome Project was started in October 1990, and then reported the sequence of the human genome in April 2003,[4] although the initial "finished" sequence was missing 8% of the genome consisting mostly of repetitive sequences.[5]
With advancements in technology that could handle sequencing of the many repetitive sequences found in human DNA that were not fully uncovered by the original Human Genome Project study, scientists reported the first end-to-end human genome sequence in March 2022.[6]
Origin of the term
The term genome was created in 1920 by Hans Winkler,[7] professor of botany at the University of Hamburg, Germany. The website Oxford Dictionaries and the Online Etymology Dictionary suggest the name is a blend of the words gene and chromosome.[8][9][10][11] However, see omics for a more thorough discussion. A few related -ome words already existed, such as biome and rhizome, forming a vocabulary into which genome fits systematically.[12]
Definition
It's very difficult to come up with a precise definition of "genome." It usually refers to the DNA (or sometimes RNA) molecules that carry the genetic information in an organism but sometimes it is difficult to decide which molecules to include in the definition; for example, bacteria usually have one or two large DNA molecules (chromosomes) that contain all of the essential genetic material but they also contain smaller extrachromosomal plasmid molecules that carry important genetic information. The definition of 'genome' that's commonly used in the scientific literature is usually restricted to the large chromosomal DNA molecules in bacteria.[13]
Eukaryotic genomes are even more difficult to define because almost all eukaryotic species contain nuclear chromosomes plus extra DNA molecules in the mitochondria. In addition, algae and plants have chloroplast DNA. Most textbooks make a distinction between the nuclear genome and the organelle (mitochondria and chloroplast) genomes so when they speak of, say, the human genome, they are only referring to the genetic material in the nucleus.[2][14] This is the most common use of 'genome' in the scientific literature.
Most eukaryotes are diploid, meaning that there are two copies of each chromosome in the nucleus but the 'genome' refers to only one copy of each chromosome. Some eukaryotes have distinctive sex chromosomes such as the X and Y chromosomes of mammals so the technical definition of the genome must include both copies of the sex chromosomes. When referring to the standard reference genome of humans, for example, it consists of one copy of each of the 22 autosomes plus one X chromosome and one Y chromosome.[15]
Sequencing and mapping
A genome sequence is the complete list of the nucleotides (A, C, G, and T for DNA genomes) that make up all the chromosomes of an individual or a species. Within a species, the vast majority of nucleotides are identical between individuals, but sequencing multiple individuals is necessary to understand the genetic diversity.

In 1976, Walter Fiers at the University of Ghent (Belgium) was the first to establish the complete nucleotide sequence of a viral RNA-genome (Bacteriophage MS2). The next year, Fred Sanger completed the first DNA-genome sequence: Phage Φ-X174, of 5386 base pairs.[16] The first bacterial genome to be sequenced was that of Haemophilus influenzae, completed by a team at The Institute for Genomic Research in 1995. A few months later, the first eukaryotic genome was completed, with sequences of the 16 chromosomes of budding yeast Saccharomyces cerevisiae published as the result of a European-led effort begun in the mid-1980s. The first genome sequence for an archaeon, Methanococcus jannaschii, was completed in 1996, again by The Institute for Genomic Research.[citation needed]
The development of new technologies has made genome sequencing dramatically cheaper and easier, and the number of complete genome sequences is growing rapidly. The US National Institutes of Health maintains one of several comprehensive databases of genomic information.[17] Among the thousands of completed genome sequencing projects include those for rice, a mouse, the plant Arabidopsis thaliana, the puffer fish, and the bacteria E. coli. In December 2013, scientists first sequenced the entire genome of a Neanderthal, an extinct species of humans. The genome was extracted from the toe bone of a 130,000-year-old Neanderthal found in a Siberian cave.[18][19]
New sequencing technologies, such as massive parallel sequencing have also opened up the prospect of personal genome sequencing as a diagnostic tool, as pioneered by Manteia Predictive Medicine. A major step toward that goal was the completion in 2007 of the full genome of James D. Watson, one of the co-discoverers of the structure of DNA.[20]
Whereas a genome sequence lists the order of every DNA base in a genome, a genome map identifies the landmarks. A genome map is less detailed than a genome sequence and aids in navigating around the genome. The Human Genome Project was organized to map and to sequence the human genome. A fundamental step in the project was the release of a detailed genomic map by Jean Weissenbach and his team at the Genoscope in Paris.[21][22]
Reference genome sequences and maps continue to be updated, removing errors and clarifying regions of high allelic complexity.[23] The decreasing cost of genomic mapping has permitted genealogical sites to offer it as a service,[24] to the extent that one may submit one's genome to crowdsourced scientific endeavours such as DNA.LAND at the New York Genome Center,[25] an example both of the economies of scale and of citizen science.[26]
Viral genomes
Viral genomes can be composed of either RNA or DNA. The genomes of RNA viruses can be either single-stranded RNA or double-stranded RNA, and may contain one or more separate RNA molecules (segments: monopartit or multipartit genome). DNA viruses can have either single-stranded or double-stranded genomes. Most DNA virus genomes are composed of a single, linear molecule of DNA, but some are made up of a circular DNA molecule.[27]
Prokaryotic genomes
Prokaryotes and eukaryotes have DNA genomes. Archaea and most bacteria have a single circular chromosome,[28] however, some bacterial species have linear or multiple chromosomes.[29][30] If the DNA is replicated faster than the bacterial cells divide, multiple copies of the chromosome can be present in a single cell, and if the cells divide faster than the DNA can be replicated, multiple replication of the chromosome is initiated before the division occurs, allowing daughter cells to inherit complete genomes and already partially replicated chromosomes. Most prokaryotes have very little repetitive DNA in their genomes.[31] However, some symbiotic bacteria (e.g. Serratia symbiotica) have reduced genomes and a high fraction of pseudogenes: only ~40% of their DNA encodes proteins.[32][33]
Some bacteria have auxiliary genetic material, also part of their genome, which is carried in plasmids. For this, the word genome should not be used as a synonym of chromosome.
Eukaryotic genomes
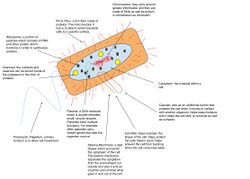
Eukaryotic genomes are composed of one or more linear DNA chromosomes. The number of chromosomes varies widely from Jack jumper ants and an asexual nemotode,[34] which each have only one pair, to a fern species that has 720 pairs.[35] It is surprising the amount of DNA that eukaryotic genomes contain compared to other genomes. The amount is even more than what is necessary for DNA protein-coding and noncoding genes due to the fact that eukaryotic genomes show as much as 64,000-fold variation in their sizes.[36] However, this special characteristic is caused by the presence of repetitive DNA, and transposable elements (TEs).
A typical human cell has two copies of each of 22 autosomes, one inherited from each parent, plus two sex chromosomes, making it diploid. Gametes, such as ova, sperm, spores, and pollen, are haploid, meaning they carry only one copy of each chromosome. In addition to the chromosomes in the nucleus, organelles such as the chloroplasts and mitochondria have their own DNA. Mitochondria are sometimes said to have their own genome often referred to as the "mitochondrial genome". The DNA found within the chloroplast may be referred to as the "plastome". Like the bacteria they originated from, mitochondria and chloroplasts have a circular chromosome.
Unlike prokaryotes where exon-intron organization of protein coding genes exists but is rather exceptional, eukaryotes generally have these features in their genes and their genomes contain variable amounts of repetitive DNA. In mammals and plants, the majority of the genome is composed of repetitive DNA.[37] Genes in eukaryotic genomes can be annotated using FINDER.[38] [39]
DNA sequencing
High-throughput technology makes sequencing to assemble new genomes accessible to everyone. Sequence polymorphisms are typically discovered by comparing resequenced isolates to a reference, whereas analyses of coverage depth and mapping topology can provide details regarding structural variations such as chromosomal translocations and segmental duplications.
Coding sequences
DNA sequences that carry the instructions to make proteins are referred to as coding sequences. The proportion of the genome occupied by coding sequences varies widely. A larger genome does not necessarily contain more genes, and the proportion of non-repetitive DNA decreases along with increasing genome size in complex eukaryotes.[37]
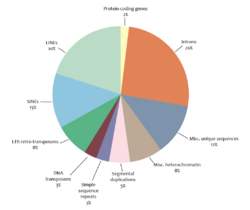
Noncoding sequences
Noncoding sequences include introns, sequences for non-coding RNAs, regulatory regions, and repetitive DNA. Noncoding sequences make up 98% of the human genome. There are two categories of repetitive DNA in the genome: tandem repeats and interspersed repeats.[40]
Tandem repeats
Short, non-coding sequences that are repeated head-to-tail are called tandem repeats. Microsatellites consisting of 2–5 basepair repeats, while minisatellite repeats are 30–35 bp. Tandem repeats make up about 4% of the human genome and 9% of the fruit fly genome.[41] Tandem repeats can be functional. For example, telomeres are composed of the tandem repeat TTAGGG in mammals, and they play an important role in protecting the ends of the chromosome.
In other cases, expansions in the number of tandem repeats in exons or introns can cause disease.[42] For example, the human gene huntingtin (Htt) typically contains 6–29 tandem repeats of the nucleotides CAG (encoding a polyglutamine tract). An expansion to over 36 repeats results in Huntington's disease, a neurodegenerative disease. Twenty human disorders are known to result from similar tandem repeat expansions in various genes. The mechanism by which proteins with expanded polygulatamine tracts cause death of neurons is not fully understood. One possibility is that the proteins fail to fold properly and avoid degradation, instead accumulating in aggregates that also sequester important transcription factors, thereby altering gene expression.[42]
Tandem repeats are usually caused by slippage during replication, unequal crossing-over and gene conversion.[43]
Transposable elements
Transposable elements (TEs) are sequences of DNA with a defined structure that are able to change their location in the genome.[41][31][44] TEs are categorized as either as a mechanism that replicates by copy-and-paste or as a mechanism that can be excised from the genome and inserted at a new location. In the human genome, there are three important classes of TEs that make up more than 45% of the human DNA; these classes are The long interspersed nuclear elements (LINEs), The interspersed nuclear elements (SINEs), and endogenous retroviruses. These elements have a big potential to modify the genetic control in a host organism.[36]
The movement of TEs is a driving force of genome evolution in eukaryotes because their insertion can disrupt gene functions, homologous recombination between TEs can produce duplications, and TE can shuffle exons and regulatory sequences to new locations.[45]
Retrotransposons
Retrotransposons[46] are found mostly in eukaryotes but not found in prokaryotes. Retrotransposons form a large portion of the genomes of many eukaryotes. A retrotransposon is a transposable element that transposes through an RNA intermediate. Retrotransposons[47] are composed of DNA, but are transcribed into RNA for transposition, then the RNA transcript is copied back to DNA formation with the help of a specific enzyme called reverse transcriptase. A retrotransposon that carries reverse transcriptase in its sequence can trigger its own transposition but retrotransposons that lack a reverse transcriptase must use reverse transcriptase synthesized by another retrotransposon. Retrotransposons can be transcribed into RNA, which are then duplicated at another site into the genome.[48] Retrotransposons can be divided into long terminal repeats (LTRs) and non-long terminal repeats (Non-LTRs).[45]
Long terminal repeats (LTRs) are derived from ancient retroviral infections, so they encode proteins related to retroviral proteins including gag (structural proteins of the virus), pol (reverse transcriptase and integrase), pro (protease), and in some cases env (envelope) genes.[44] These genes are flanked by long repeats at both 5' and 3' ends. It has been reported that LTRs consist of the largest fraction in most plant genome and might account for the huge variation in genome size.[49]
Non-long terminal repeats (Non-LTRs) are classified as long interspersed nuclear elements (LINEs), short interspersed nuclear elements (SINEs), and Penelope-like elements (PLEs). In Dictyostelium discoideum, there is another DIRS-like elements belong to Non-LTRs. Non-LTRs are widely spread in eukaryotic genomes.[50]
Long interspersed elements (LINEs) encode genes for reverse transcriptase and endonuclease, making them autonomous transposable elements. The human genome has around 500,000 LINEs, taking around 17% of the genome.[51]
Short interspersed elements (SINEs) are usually less than 500 base pairs and are non-autonomous, so they rely on the proteins encoded by LINEs for transposition.[52] The Alu element is the most common SINE found in primates. It is about 350 base pairs and occupies about 11% of the human genome with around 1,500,000 copies.[45]
DNA transposons
DNA transposons encode a transposase enzyme between inverted terminal repeats. When expressed, the transposase recognizes the terminal inverted repeats that flank the transposon and catalyzes its excision and reinsertion in a new site.[41] This cut-and-paste mechanism typically reinserts transposons near their original location (within 100kb).[45] DNA transposons are found in bacteria and make up 3% of the human genome and 12% of the genome of the roundworm C. elegans.[45]
Genome size
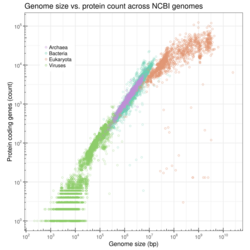
Genome size is the total number of the DNA base pairs in one copy of a haploid genome. Genome size varies widely across species. Invertebrates have small genomes, this is also correlated to a small number of transposable elements. Fish and Amphibians have intermediate-size genomes, and birds have relatively small genomes but it has been suggested that birds lost a substantial portion of their genomes during the phase of transition to flight. Before this loss, DNA methylation allows the adequate expansion of the genome.[36]
In humans, the nuclear genome comprises approximately 3.1 billion nucleotides of DNA, divided into 24 linear molecules, the shortest 45 000 000 nucleotides in length and the longest 248 000 000 nucleotides, each contained in a different chromosome.[53] There is no clear and consistent correlation between morphological complexity and genome size in either prokaryotes or lower eukaryotes.[37][54] Genome size is largely a function of the expansion and contraction of repetitive DNA elements.
Since genomes are very complex, one research strategy is to reduce the number of genes in a genome to the bare minimum and still have the organism in question survive. There is experimental work being done on minimal genomes for single cell organisms as well as minimal genomes for multi-cellular organisms (see developmental biology). The work is both in vivo and in silico.[55][56]
Genome size differences due to transposable elements

There are many enormous differences in size in genomes, specially mentioned before in the multicellular eukaryotic genomes. Much of this is due to the differing abundances of transposable elements, which evolve by creating new copies of themselves in the chromosomes.[36] Eukaryote genomes often contain many thousands of copies of these elements, most of which have acquired mutations that make them defective. Here is a table of some significant or representative genomes. See #See also for lists of sequenced genomes.
| Organism type | Organism | Genome size (base pairs) |
Approx. no. of genes | Note | |
|---|---|---|---|---|---|
| Virus | Porcine circovirus type 1 | 1,759 | 1.8 kB | Smallest viruses replicating autonomously in eukaryotic cells[57] | |
| Virus | Bacteriophage MS2 | 3,569 | 3.6 kB | First sequenced RNA-genome[58] | |
| Virus | SV40 | 5,224 | 5.2 kB | [59] | |
| Virus | Phage Φ-X174 | 5,386 | 5.4 kB | First sequenced DNA-genome[60] | |
| Virus | HIV | 9,749 | 9.7 kB | [61] | |
| Virus | Phage λ | 48,502 | 48.5 kB | Often used as a vector for the cloning of recombinant DNA | |
| Virus | Megavirus | 1,259,197 | 1.3 MB | Until 2013 the largest known viral genome[65] | |
| Virus | Pandoravirus salinus | 2,470,000 | 2.47 MB | Largest known viral genome.[66] | |
| Eukaryotic organelle | Human mitochondrion | 16,569 | 16.6 kB | [67] | |
| Bacterium | Nasuia deltocephalinicola (strain NAS-ALF) | 112,091 | 112 kB | 137 | Smallest known non-viral genome. Symbiont of leafhoppers.[68] |
| Bacterium | Carsonella ruddii | 159,662 | 160 kB | An endosymbiont of psyllid insects | |
| Bacterium | Buchnera aphidicola | 600,000 | 600 kB | An endosymbiont of aphids[69] | |
| Bacterium | Wigglesworthia glossinidia | 700,000 | 700 kB | A symbiont in the gut of the tsetse fly | |
| Bacterium – cyanobacterium | Prochlorococcus spp. (1.7 Mb) | 1,700,000 | 1.7 MB | 1,884 | Smallest known cyanobacterium genome. One of the primary photosynthesizers on Earth.[70][71] |
| Bacterium | Haemophilus influenzae | 1,830,000 | 1.8 MB | First genome of a living organism sequenced, July 1995[72] | |
| Bacterium | Escherichia coli | 4,600,000 | 4.6 MB | 4,288 | [73] |
| Bacterium – cyanobacterium | Nostoc punctiforme | 9,000,000 | 9 MB | 7,432 | 7432 open reading frames[74] |
| Bacterium | Solibacter usitatus (strain Ellin 6076) | 9,970,000 | 10 MB | [75] | |
| Amoeboid | Polychaos dubium ("Amoeba" dubia) | 670,000,000,000 | 670 GB | Largest known genome.[76] (Disputed)[77] | |
| Plant | Genlisea tuberosa | 61,000,000 | 61 MB | Smallest recorded flowering plant genome, 2014[78] | |
| Plant | Arabidopsis thaliana | 135,000,000[79] | 135 MB | 27,655[80] | First plant genome sequenced, December 2000[81] |
| Plant | Populus trichocarpa | 480,000,000 | 480 MB | 73,013 | First tree genome sequenced, September 2006[82] |
| Plant | Pinus taeda (Loblolly pine) | 22,180,000,000 | 22.18 GB | 50,172 | Gymnosperms generally have much larger genomes than angiosperms[83][84] |
| Plant | Fritillaria assyriaca | 130,000,000,000 | 130 GB | ||
| Plant | Paris japonica (Japanese-native, order Liliales) | 150,000,000,000 | 150 GB | Largest plant genome known[85] | |
| Plant – moss | Physcomitrella patens | 480,000,000 | 480 MB | First genome of a bryophyte sequenced, January 2008[86] | |
| Fungus – yeast | Saccharomyces cerevisiae | 12,100,000 | 12.1 MB | 6,294 | First eukaryotic genome sequenced, 1996[87] |
| Fungus | Aspergillus nidulans | 30,000,000 | 30 MB | 9,541 | [88] |
| Nematode | Pratylenchus coffeae | 20,000,000 | 20 MB | [89] Smallest animal genome known[90] | |
| Nematode | Caenorhabditis elegans | 100,300,000 | 100 MB | 19,000 | First multicellular animal genome sequenced, December 1998[91] |
| Insect | Belgica antarctica (Antarctic midge) | 99,000,000 | 99 MB | Smallest insect genome sequenced thus far, likely an adaptation to an extreme environment[92] | |
| Insect | Drosophila melanogaster (fruit fly) | 175,000,000 | 175 MB | 13,600 | Size variation based on strain (175–180 Mb; standard y w strain is 175 Mb)[93] |
| Insect | Apis mellifera (honey bee) | 236,000,000 | 236 MB | 10,157 | [94] |
| Insect | Bombyx mori (silk moth) | 432,000,000 | 432 MB | 14,623 | 14,623 predicted genes[95] |
| Insect | Solenopsis invicta (fire ant) | 480,000,000 | 480 MB | 16,569 | [96] |
| Crustacean | Antarctic krill | 48,010,000,000 | 48 GB | 23,000 | 70-92% repetitive DNA[97] |
| Amphibian | Neuse River waterdog | 118,000,000,000 | 118 GB | Largest tetrapod genome sequenced as of 2022[98] | |
| Amphibian | Ornate burrowing frog | 1,060,000,000 | 1.06 GB | Smallest known frog genome[99] | |
| Mammal | Mus musculus | 2,700,000,000 | 2.7 GB | 20,210 | [100] |
| Mammal | Pan paniscus | 3,286,640,000 | 3.3 GB | 20,000 | Bonobo – estimated genome size 3.29 billion bp[101] |
| Mammal | Homo sapiens | 3,117,000,000 | 3.1 GB | 20,000 | Homo sapiens genome size estimated at 3.12 Gbp in 2022[53]
Initial sequencing and analysis of the human genome[102] |
| Bird | Gallus gallus | 1,043,000,000 | 1.0 GB | 20,000 | [103] |
| Fish | Tetraodon nigroviridis (type of puffer fish) | 385,000,000 | 390 MB | Smallest vertebrate genome known, estimated to be 340 Mb[104][105] – 385 Mb[106] | |
| Fish | Protopterus aethiopicus (marbled lungfish) | 130,000,000,000 | 130 GB | Largest vertebrate genome known | |
Genomic alterations
All the cells of an organism originate from a single cell, so they are expected to have identical genomes; however, in some cases, differences arise. Both the process of copying DNA during cell division and exposure to environmental mutagens can result in mutations in somatic cells. In some cases, such mutations lead to cancer because they cause cells to divide more quickly and invade surrounding tissues.[107] In certain lymphocytes in the human immune system, V(D)J recombination generates different genomic sequences such that each cell produces a unique antibody or T cell receptors.
During meiosis, diploid cells divide twice to produce haploid germ cells. During this process, recombination results in a reshuffling of the genetic material from homologous chromosomes so each gamete has a unique genome.
Genome-wide reprogramming
Genome-wide reprogramming in mouse primordial germ cells involves epigenetic imprint erasure leading to totipotency. Reprogramming is facilitated by active DNA demethylation, a process that entails the DNA base excision repair pathway.[108] This pathway is employed in the erasure of CpG methylation (5mC) in primordial germ cells. The erasure of 5mC occurs via its conversion to 5-hydroxymethylcytosine (5hmC) driven by high levels of the ten-eleven dioxygenase enzymes TET1 and TET2.[109]
Genome evolution
Genomes are more than the sum of an organism's genes and have traits that may be measured and studied without reference to the details of any particular genes and their products. Researchers compare traits such as karyotype (chromosome number), genome size, gene order, codon usage bias, and GC-content to determine what mechanisms could have produced the great variety of genomes that exist today (for recent overviews, see Brown 2002; Saccone and Pesole 2003; Benfey and Protopapas 2004; Gibson and Muse 2004; Reese 2004; Gregory 2005).
Duplications play a major role in shaping the genome. Duplication may range from extension of short tandem repeats, to duplication of a cluster of genes, and all the way to duplication of entire chromosomes or even entire genomes. Such duplications are probably fundamental to the creation of genetic novelty.
Horizontal gene transfer is invoked to explain how there is often an extreme similarity between small portions of the genomes of two organisms that are otherwise very distantly related. Horizontal gene transfer seems to be common among many microbes. Also, eukaryotic cells seem to have experienced a transfer of some genetic material from their chloroplast and mitochondrial genomes to their nuclear chromosomes. Recent empirical data suggest an important role of viruses and sub-viral RNA-networks to represent a main driving role to generate genetic novelty and natural genome editing.
In fiction
Works of science fiction illustrate concerns about the availability of genome sequences.
Michael Crichton's 1990 novel Jurassic Park and the subsequent film tell the story of a billionaire who creates a theme park of cloned dinosaurs on a remote island, with disastrous outcomes. A geneticist extracts dinosaur DNA from the blood of ancient mosquitoes and fills in the gaps with DNA from modern species to create several species of dinosaurs. A chaos theorist is asked to give his expert opinion on the safety of engineering an ecosystem with the dinosaurs, and he repeatedly warns that the outcomes of the project will be unpredictable and ultimately uncontrollable. These warnings about the perils of using genomic information are a major theme of the book.
The 1997 film Gattaca is set in a futurist society where genomes of children are engineered to contain the most ideal combination of their parents' traits, and metrics such as risk of heart disease and predicted life expectancy are documented for each person based on their genome. People conceived outside of the eugenics program, known as "In-Valids" suffer discrimination and are relegated to menial occupations. The protagonist of the film is an In-Valid who works to defy the supposed genetic odds and achieve his dream of working as a space navigator. The film warns against a future where genomic information fuels prejudice and extreme class differences between those who can and cannot afford genetically engineered children.[110]
See also
- Bacterial genome size
- Cryoconservation of animal genetic resources
- Genome Browser
- Genome Compiler
- Genome topology
- Genome-wide association study
- List of sequenced animal genomes
- List of sequenced archaeal genomes
- List of sequenced bacterial genomes
- List of sequenced eukaryotic genomes
- List of sequenced fungi genomes
- List of sequenced plant genomes
- List of sequenced plastomes
- List of sequenced protist genomes
- Metagenomics
- Microbiome
- Molecular epidemiology
- Molecular pathological epidemiology
- Molecular pathology
- Nucleic acid sequence
- Pan-genome
- Precision medicine
- Regulator gene
- Whole genome sequencing
References
- ↑ Roth, Stephanie Clare (2019-07-01). "What is genomic medicine?". Journal of the Medical Library Association (University Library System, University of Pittsburgh) 107 (3): 442–448. doi:10.5195/jmla.2019.604. ISSN 1558-9439. PMID 31258451.
- ↑ 2.0 2.1 2.2 Graur, Dan; Sater, Amy K.; Cooper, Tim F. (2016). Molecular and Genome Evolution. Sinauer Associates, Inc.. ISBN 9781605354699. OCLC 951474209. https://books.google.com/books?id=blOZjgEACAAJ.
- ↑ Brosius, J (2009). "The Fragmented Gene". Annals of the New York Academy of Sciences 1178 (1): 186–93. doi:10.1111/j.1749-6632.2009.05004.x. PMID 19845638. Bibcode: 2009NYASA1178..186B. https://nyaspubs.onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.2009.05004.x.
- ↑ "The Human Genome Project". https://www.genome.gov/human-genome-project.
- ↑ "First complete sequence of a human genome". 2022-04-11. https://www.nih.gov/news-events/nih-research-matters/first-complete-sequence-human-genome#:~:text=This%20last%208%25%20of%20the,of%20each%20chromosome..
- ↑ Hartley, Gabrielle (31 March 2022). "The Human Genome Project pieced together only 92% of the DNA – now scientists have finally filled in the remaining 8%". The Conversation US, Inc.. https://theconversation.com/the-human-genome-project-pieced-together-only-92-of-the-dna-now-scientists-have-finally-filled-in-the-remaining-8-176138.
- ↑ Verbreitung und Ursache der Parthenogenesis im Pflanzen- und Tierreiche. Jena: Verlag Fischer. 1920. https://archive.org/details/verbreitungundur00wink.
- ↑ "definition of Genome in Oxford dictionary". http://www.oxforddictionaries.com/us/definition/american_english/genome.
- ↑ genome (3rd ed.), Oxford University Press, September 2005, http://oed.com/search?searchType=dictionary&q=genome (Subscription or UK public library membership required.)
- ↑ "genome". genome. Oxford University Press. http://www.lexico.com/definition/genome.
- ↑ Template:Cite OEtymD
- ↑ Lederberg, Joshua; McCray, Alexa T. (2001). "'Ome Sweet 'Omics – A Genealogical Treasury of Words". The Scientist 15 (7). http://lhncbc.nlm.nih.gov/lhc/docs/published/2001/pub2001047.pdf.
- ↑ "The ingenuity of bacterial genomes". Annual Review of Microbiology 74: 815–834. 2020. doi:10.1146/annurev-micro-020518-115822. PMID 32692614.
- ↑ Genomes 4. New York, NY, USA: Garland Science. 2018. ISBN 9780815345084.
- ↑ "Ensembl Human Assembly and gene annotation (GRCh38)". Ensembl. https://useast.ensembl.org/Homo_sapiens/Info/Annotation.
- ↑ "All about genes". http://www.beowulf.org.uk/.
- ↑ "Genome Home". 2010-12-08. https://www.ncbi.nlm.nih.gov/sites/entrez?db=Genome&itool=toolbar.
- ↑ Zimmer, Carl (18 December 2013). "Toe Fossil Provides Complete Neanderthal Genome". The New York Times. https://www.nytimes.com/2013/12/19/science/toe-fossil-provides-complete-neanderthal-genome.html.
- ↑ "The complete genome sequence of a Neanderthal from the Altai Mountains". Nature 505 (7481): 43–49. January 2014. doi:10.1038/nature12886. PMID 24352235. Bibcode: 2014Natur.505...43P.
- ↑ Wade, Nicholas (2007-05-31). "Genome of DNA Pioneer Is Deciphered". The New York Times. https://www.nytimes.com/2007/05/31/science/31cnd-gene.html.
- ↑ "What's a Genome?". Genomenewsnetwork.org. 2003-01-15. http://www.genomenewsnetwork.org/resources/whats_a_genome/Chp3_1.shtml.
- ↑ "Mapping Factsheet". 2004-03-29. https://www.ncbi.nlm.nih.gov/About/primer/mapping.html.
- ↑ Genome Reference Consortium. "Assembling the Genome". https://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/info/.
- ↑ Kaplan, Sarah (2016-04-17). "How do your 20,000 genes determine so many wildly different traits? They multitask.". The Washington Post. https://www.washingtonpost.com/news/speaking-of-science/wp/2016/05/17/how-do-your-20000-genes-determine-so-many-wildly-different-traits-they-multitask/.
- ↑ Check Hayden, Erika (2015). "Scientists hope to attract millions to 'DNA.LAND'". Nature. doi:10.1038/nature.2015.18514. https://www.nature.com/news/scientists-hope-to-attract-millions-to-dna-land-1.18514.
- ↑ Zimmer, Carl (25 July 2016). "Game of Genomes, Episode 13: Answers and Questions". STAT. https://www.statnews.com/feature/game-of-genomes/season-three/.
- ↑ Gelderblom, Hans R. (1996). Structure and Classification of Viruses (4th ed.). Galveston, TX: The University of Texas Medical Branch at Galveston. ISBN 9780963117212. https://www.ncbi.nlm.nih.gov/books/NBK8174/.
- ↑ "Archaeal chromosome biology". Journal of Molecular Microbiology and Biotechnology 24 (5–6): 420–27. 2014. doi:10.1159/000368854. PMID 25732343.
- ↑ Chaconas, George; Chen, Carton W. (2005). "Replication of Linear Bacterial Chromosomes: No Longer Going Around in Circles". The Bacterial Chromosome. pp. 525–540. doi:10.1128/9781555817640.ch29. ISBN 9781555812324. http://www.asmscience.org/content/book/10.1128/9781555817640.chap29.
- ↑ "Bacterial Chromosomes". 2002. http://www.sci.sdsu.edu/~smaloy/MicrobialGenetics/topics/chroms-genes-prots/chromosomes.html.
- ↑ 31.0 31.1 "Constraints and plasticity in genome and molecular-phenome evolution". Nature Reviews. Genetics 11 (7): 487–98. July 2010. doi:10.1038/nrg2810. PMID 20548290.
- ↑ "Extreme genome reduction in symbiotic bacteria". Nature Reviews. Microbiology 10 (1): 13–26. November 2011. doi:10.1038/nrmicro2670. PMID 22064560.
- ↑ "Insights from 20 years of bacterial genome sequencing". Functional & Integrative Genomics 15 (2): 141–61. March 2015. doi:10.1007/s10142-015-0433-4. PMID 25722247.
- ↑ "Scientists sequence asexual tiny worm whose lineage stretches back 18 million years". https://www.sciencedaily.com/releases/2017/09/170921141303.htm.
- ↑ Khandelwal, Sharda (March 1990). "Chromosome evolution in the genus Ophioglossum L.". Botanical Journal of the Linnean Society 102 (3): 205–17. doi:10.1111/j.1095-8339.1990.tb01876.x.
- ↑ 36.0 36.1 36.2 36.3 Zhou, Wanding; Liang, Gangning; Molloy, Peter L.; Jones, Peter A. (11 August 2020). "DNA methylation enables transposable element-driven genome expansion". Proceedings of the National Academy of Sciences of the United States of America 117 (32): 19359–19366. doi:10.1073/pnas.1921719117. ISSN 1091-6490. PMID 32719115. Bibcode: 2020PNAS..11719359Z.
- ↑ 37.0 37.1 37.2 Lewin, Benjamin (2004). Genes VIII (8th ed.). Upper Saddle River, NJ: Pearson/Prentice Hall. ISBN 978-0-13-143981-8.
- ↑ "FINDER: an automated software package to annotate eukaryotic genes from RNA-Seq data and associated protein sequences". BMC Bioinformatics 44 (9): e89. Apr 2021. doi:10.1186/s12859-021-04120-9. PMID 33879057.
- ↑ Harb, Omar S.; Boehme, Ulrike; Crouch, Kathryn; Ifeonu, Olukemi O.; Roos, David S.; Silva, Joana C.; Silva-Franco, Fatima; Svärd, Staffan et al. (2016). Genomes. Wellcome Trust–Funded Monographs and Book Chapters. Springer. PMID 32348078. https://www.ncbi.nlm.nih.gov/books/NBK556349/#genomes.s1.
- ↑ Stojanovic, Nikola, ed (2007). Computational genomics : current methods. Wymondham: Horizon Bioscience. ISBN 978-1-904933-30-4.
- ↑ 41.0 41.1 41.2 "Repeat DNA in genome organization and stability". Current Opinion in Genetics & Development 31: 12–19. April 2015. doi:10.1016/j.gde.2015.03.009. PMID 25917896.
- ↑ 42.0 42.1 "The biological effects of simple tandem repeats: lessons from the repeat expansion diseases". Genome Research 18 (7): 1011–19. July 2008. doi:10.1101/gr.070409.107. PMID 18593815.
- ↑ "Microsatellites: genomic distribution, putative functions and mutational mechanisms: a review". Molecular Ecology 11 (12): 2453–65. December 2002. doi:10.1046/j.1365-294X.2002.01643.x. PMID 12453231.
- ↑ 44.0 44.1 "Transposable elements and the evolution of eukaryotic genomes". Proceedings of the National Academy of Sciences of the United States of America 103 (47): 17600–01. November 2006. doi:10.1073/pnas.0607612103. PMID 17101965. Bibcode: 2006PNAS..10317600W.
- ↑ 45.0 45.1 45.2 45.3 45.4 "Mobile elements: drivers of genome evolution". Science 303 (5664): 1626–32. March 2004. doi:10.1126/science.1089670. PMID 15016989. Bibcode: 2004Sci...303.1626K.
- ↑ "Transposon | genetics". https://www.britannica.com/science/transposon.
- ↑ Sanders, Mark Frederick (2019). Genetic Analysis: an integrated approach third edition. New York: Pearson, always learning, and mastering. pp. 425. ISBN 9780134605173.
- ↑ "Mobile elements and mammalian genome evolution". Current Opinion in Genetics & Development 13 (6): 651–58. December 2003. doi:10.1016/j.gde.2003.10.013. PMID 14638329.
- ↑ "Transposable elements and host genome evolution". Trends in Ecology & Evolution 15 (3): 95–99. March 2000. doi:10.1016/S0169-5347(99)01817-0. PMID 10675923.
- ↑ "Comparative genomics and molecular dynamics of DNA repeats in eukaryotes". Microbiology and Molecular Biology Reviews 72 (4): 686–727. December 2008. doi:10.1128/MMBR.00011-08. PMID 19052325.
- ↑ "The impact of retrotransposons on human genome evolution". Nature Reviews. Genetics 10 (10): 691–703. October 2009. doi:10.1038/nrg2640. PMID 19763152.
- ↑ "LINE-1 retrotransposons: modulators of quantity and quality of mammalian gene expression?". BioEssays 27 (8): 775–84. August 2005. doi:10.1002/bies.20257. PMID 16015595.
- ↑ 53.0 53.1 Nurk, Sergey et al. (2022-03-31). "The complete sequence of a human genome". Science 376 (6588): 44–53. doi:10.1126/science.abj6987. PMID 35357919. PMC 9186530. Bibcode: 2022Sci...376...44N. https://eprints.iisc.ac.in/71762/1/Sci_376-6588_44-531_2022%20.pdf.
- ↑ "Eukaryotic genome size databases". Nucleic Acids Research 35 (Database issue): D332–38. January 2007. doi:10.1093/nar/gkl828. PMID 17090588.
- ↑ "Essential genes of a minimal bacterium". Proceedings of the National Academy of Sciences of the United States of America 103 (2): 425–30. January 2006. doi:10.1073/pnas.0510013103. PMID 16407165. Bibcode: 2006PNAS..103..425G.
- ↑ "Towards synthesis of a minimal cell". Molecular Systems Biology 2 (1): 45. 2006. doi:10.1038/msb4100090. PMID 16924266.
- ↑ Mankertz P (2008). "Molecular Biology of Porcine Circoviruses". Animal Viruses: Molecular Biology. Caister Academic Press. ISBN 978-1-904455-22-6. http://www.horizonpress.com/avir.
- ↑ "Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene". Nature 260 (5551): 500–07. April 1976. doi:10.1038/260500a0. PMID 1264203. Bibcode: 1976Natur.260..500F.
- ↑ "Complete nucleotide sequence of SV40 DNA". Nature 273 (5658): 113–20. May 1978. doi:10.1038/273113a0. PMID 205802. Bibcode: 1978Natur.273..113F.
- ↑ "Nucleotide sequence of bacteriophage phi X174 DNA". Nature 265 (5596): 687–95. February 1977. doi:10.1038/265687a0. PMID 870828. Bibcode: 1977Natur.265..687S.
- ↑ "Virology – Human Immunodeficiency Virus And Aids, Structure: The Genome And Proteins of HIV". Pathmicro.med.sc.edu. 2010-07-01. http://pathmicro.med.sc.edu/lecture/hiv9.htm.
- ↑ "Recombineering: Genetic Engineering in Bacteria Using Homologous Recombination". Current Protocols in Molecular Biology Chapter 1: Unit 1.16. April 2007. doi:10.1002/0471142727.mb0116s78. ISBN 978-0-471-14272-0. PMID 18265390.
- ↑ "A new look at bacteriophage lambda genetic networks". Journal of Bacteriology 189 (2): 298–304. January 2007. doi:10.1128/JB.01215-06. PMID 17085553.
- ↑ "Nucleotide sequence of bacteriophage lambda DNA". Journal of Molecular Biology 162 (4): 729–73. December 1982. doi:10.1016/0022-2836(82)90546-0. PMID 6221115.
- ↑ "Genomics of Megavirus and the elusive fourth domain of Life". Communicative & Integrative Biology 5 (1): 102–06. January 2012. doi:10.4161/cib.18624. PMID 22482024.
- ↑ "Pandoraviruses: amoeba viruses with genomes up to 2.5 Mb reaching that of parasitic eukaryotes". Science 341 (6143): 281–86. July 2013. doi:10.1126/science.1239181. PMID 23869018. Bibcode: 2013Sci...341..281P. https://hal-cea.archives-ouvertes.fr/cea-00862677/file/phi.pdf.
- ↑ "Sequence and organization of the human mitochondrial genome". Nature 290 (5806): 457–65. April 1981. doi:10.1038/290457a0. PMID 7219534. Bibcode: 1981Natur.290..457A.
- ↑ "Small, smaller, smallest: the origins and evolution of ancient dual symbioses in a Phloem-feeding insect". Genome Biology and Evolution 5 (9): 1675–88. 5 August 2013. doi:10.1093/gbe/evt118. PMID 23918810.
- ↑ "Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS". Nature 407 (6800): 81–86. September 2000. doi:10.1038/35024074. PMID 10993077. Bibcode: 2000Natur.407...81S.
- ↑ "Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation". Nature 424 (6952): 1042–47. August 2003. doi:10.1038/nature01947. PMID 12917642. Bibcode: 2003Natur.424.1042R.
- ↑ "Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome". Proceedings of the National Academy of Sciences of the United States of America 100 (17): 10020–25. August 2003. doi:10.1073/pnas.1733211100. PMID 12917486. Bibcode: 2003PNAS..10010020D.
- ↑ "Whole-genome random sequencing and assembly of Haemophilus influenzae Rd". Science 269 (5223): 496–512. July 1995. doi:10.1126/science.7542800. PMID 7542800. Bibcode: 1995Sci...269..496F.
- ↑ "The complete genome sequence of Escherichia coli K-12". Science 277 (5331): 1453–62. September 1997. doi:10.1126/science.277.5331.1453. PMID 9278503.
- ↑ "An overview of the genome of Nostoc punctiforme, a multicellular, symbiotic cyanobacterium". Photosynthesis Research 70 (1): 85–106. 2001. doi:10.1023/A:1013840025518. PMID 16228364.
- ↑ Steinke, Dirk, ed (15 September 2011). "Biological consequences of ancient gene acquisition and duplication in the large genome of Candidatus Solibacter usitatus Ellin6076". PLOS ONE 6 (9): e24882. doi:10.1371/journal.pone.0024882. PMID 21949776. Bibcode: 2011PLoSO...624882C.
- ↑ "The dynamic nature of eukaryotic genomes". Molecular Biology and Evolution 25 (4): 787–94. April 2008. doi:10.1093/molbev/msn032. PMID 18258610.
- ↑ ScienceShot: Biggest Genome Ever , comments: "The measurement for Amoeba dubia and other protozoa which have been reported to have very large genomes were made in the 1960s using a rough biochemical approach which is now considered to be an unreliable method for accurate genome size determinations."
- ↑ "Evolution of genome size and chromosome number in the carnivorous plant genus Genlisea (Lentibulariaceae), with a new estimate of the minimum genome size in angiosperms". Annals of Botany 114 (8): 1651–63. December 2014. doi:10.1093/aob/mcu189. PMID 25274549.
- ↑ "Genome Assembly". The Arabidopsis Information Resource (TAIR). https://www.arabidopsis.org/portals/genAnnotation/gene_structural_annotation/agicomplete.jsp.
- ↑ "Details - Arabidopsis thaliana - Ensembl Genomes 40". http://plants.ensembl.org/Arabidopsis_thaliana/Info/Annotation/.
- ↑ "Smallest angiosperm genomes found in lentibulariaceae, with chromosomes of bacterial size". Plant Biology 8 (6): 770–77. November 2006. doi:10.1055/s-2006-924101. PMID 17203433.
- ↑ "The genome of black cottonwood, Populus trichocarpa (Torr. & Gray)". Science 313 (5793): 1596–604. September 2006. doi:10.1126/science.1128691. PMID 16973872. Bibcode: 2006Sci...313.1596T. https://digital.library.unt.edu/ark:/67531/metadc883930/m2/1/high_res_d/901819.pdf.
- ↑ Zimin, Aleksey et al. (Mar 2014). "Sequencing and Assembly of the 22-Gb Loblolly Pine Genome". Genetics 196 (3): 875–890. doi:10.1534/genetics.113.159715. PMID 24653210.
- ↑ Neale, David B (Mar 2014). "Decoding the massive genome of loblolly pine using haploid DNA and novel assembly strategies". Genome Biology 15 (3): R59. doi:10.1186/gb-2014-15-3-r59. PMID 24647006.
- ↑ Pellicer, Jaume; Fay, Michael F.; Leitch, Ilia J. (15 September 2010). "The largest eukaryotic genome of them all?". Botanical Journal of the Linnean Society 164 (1): 10–15. doi:10.1111/j.1095-8339.2010.01072.x.
- ↑ "Exploring plant biodiversity: the Physcomitrella genome and beyond". Trends in Plant Science 13 (10): 542–49. October 2008. doi:10.1016/j.tplants.2008.07.002. PMID 18762443.
- ↑ "Saccharomyces Genome Database". Yeastgenome.org. http://www.yeastgenome.org/.
- ↑ "Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae". Nature 438 (7071): 1105–15. December 2005. doi:10.1038/nature04341. PMID 16372000. Bibcode: 2005Natur.438.1105G.
- ↑ "Genome size of plant-parasitic nematodes". Nematology 9 (3): 449–50. 2007. doi:10.1163/156854107781352089.
- ↑ "Animal Genome Size Database". Gregory, T.R. (2016). Animal Genome Size Database.. 2005. http://www.genomesize.com/statistics.php?stats=entire#stats_top.
- ↑ The C. elegans Sequencing Consortium (December 1998). "Genome sequence of the nematode C. elegans: a platform for investigating biology". Science 282 (5396): 2012–18. doi:10.1126/science.282.5396.2012. PMID 9851916. Bibcode: 1998Sci...282.2012..
- ↑ Kelley, Joanna L.; Peyton, Justin T.; Fiston-Lavier, Anna-Sophie; Teets, Nicholas M.; Yee, Muh-Ching; Johnston, J. Spencer; Bustamante, Carlos D.; Lee, Richard E. et al. (2014-08-12). "Compact genome of the Antarctic midge is likely an adaptation to an extreme environment". Nature Communications 5: 4611. doi:10.1038/ncomms5611. ISSN 2041-1723. PMID 25118180. Bibcode: 2014NatCo...5.4611K.
- ↑ "Intrapopulation genome size variation in D. melanogaster reflects life history variation and plasticity". PLOS Genetics 10 (7): e1004522. July 2014. doi:10.1371/journal.pgen.1004522. PMID 25057905.
- ↑ Honeybee Genome Sequencing Consortium (October 2006). "Insights into social insects from the genome of the honeybee Apis mellifera". Nature 443 (7114): 931–49. doi:10.1038/nature05260. PMID 17073008. Bibcode: 2006Natur.443..931T.
- ↑ The International Silkworm Genome (December 2008). "The genome of a lepidopteran model insect, the silkworm Bombyx mori". Insect Biochemistry and Molecular Biology 38 (12): 1036–45. doi:10.1016/j.ibmb.2008.11.004. PMID 19121390.
- ↑ "The genome of the fire ant Solenopsis invicta". Proceedings of the National Academy of Sciences of the United States of America 108 (14): 5679–84. April 2011. doi:10.1073/pnas.1009690108. PMID 21282665. Bibcode: 2011PNAS..108.5679W.
- ↑ Shao, Changwei; Sun, Shuai; Liu, Kaiqiang; Wang, Jiahao; Li, Shuo; Liu, Qun; Deagle, Bruce E.; Seim, Inge et al. (2023-03-16). "The enormous repetitive Antarctic krill genome reveals environmental adaptations and population insights". Cell Glish 186 (6): 1279–1294.e19. doi:10.1016/j.cell.2023.02.005. ISSN 0092-8674. PMID 36868220.
- ↑ "Junk DNA Deforms Salamander Bodies". https://www.scientificamerican.com/article/junk-dna-deforms-salamander-bodies/.
- ↑ A bird-like genome from a frog - PNAS
- ↑ Roberts, Richard J, ed (May 2009). "Lineage-specific biology revealed by a finished genome assembly of the mouse". PLOS Biology 7 (5): e1000112. doi:10.1371/journal.pbio.1000112. PMID 19468303.
- ↑ "Pan paniscus (pygmy chimpanzee)". nih.gov. https://www.ncbi.nlm.nih.gov/genome/10729.
- ↑ "The sequence of the human genome". Science 291 (5507): 1304–51. February 2001. doi:10.1126/science.1058040. PMID 11181995. Bibcode: 2001Sci...291.1304V.
- ↑ International Chicken Genome Sequencing Consortium (December 2004). "Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution". Nature 432 (7018): 695–716. doi:10.1038/nature03154. ISSN 0028-0836. PMID 15592404. Bibcode: 2004Natur.432..695C.
- ↑ "Characterization and repeat analysis of the compact genome of the freshwater pufferfish Tetraodon nigroviridis". Genome Research 10 (7): 939–49. July 2000. doi:10.1101/gr.10.7.939. PMID 10899143.
- ↑ "Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype". Nature 431 (7011): 946–57. October 2004. doi:10.1038/nature03025. PMID 15496914. Bibcode: 2004Natur.431..946J.
- ↑ "Tetraodon Project Information". http://www.broadinstitute.org/annotation/tetraodon/background.html.
- ↑ "Somatic mutation in cancer and normal cells". Science 349 (6255): 1483–89. September 2015. doi:10.1126/science.aab4082. PMID 26404825. Bibcode: 2015Sci...349.1483M.
- ↑ "Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway". Science 329 (5987): 78–82. July 2010. doi:10.1126/science.1187945. PMID 20595612. Bibcode: 2010Sci...329...78H.
- ↑ "Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine". Science 339 (6118): 448–52. January 2013. doi:10.1126/science.1229277. PMID 23223451. Bibcode: 2013Sci...339..448H.
- ↑ "Gattaca (movie)". Rotten Tomatoes. 24 October 1997. https://www.rottentomatoes.com/m/gattaca/.
Further reading
- Essentials of Genomics. Prentice Hall. 2004.
- Brown, Terence A. (2002). Genomes 2. Oxford: Bios Scientific Publishers. ISBN 978-1-85996-029-5.
- Gibson, Greg; Muse, Spencer V. (2004). A Primer of Genome Science (Second ed.). Sunderland, Mass: Sinauer Assoc. ISBN 978-0-87893-234-4. https://archive.org/details/primerofgenomesc00greg.
- Gregory, T. Ryan (2005). The Evolution of the Genome. Elsevier. ISBN 978-0-12-301463-4.
- Reece, Richard J. (2004). Analysis of Genes and Genomes. Chichester: John Wiley & Sons. ISBN 978-0-470-84379-6.
- Saccone, Cecilia; Pesole, Graziano (2003). Handbook of Comparative Genomics. Chichester: John Wiley & Sons. ISBN 978-0-471-39128-9.
- "In silico multicellular systems biology and minimal genomes". Drug Discovery Today 8 (24): 1121–27. December 2003. doi:10.1016/S1359-6446(03)02918-0. PMID 14678738.
External links
- UCSC Genome Browser – view the genome and annotations for more than 80 organisms.
- genomecenter.howard.edu (archived 9 August 2013)
- Build a DNA Molecule (archived 9 June 2010)
- Some comparative genome sizes
- DNA Interactive: The History of DNA Science
- DNA From The Beginning
- All About The Human Genome Project—from Genome.gov
- Animal genome size database
- Plant genome size database (archived 1 September 2005)
- GOLD:Genomes OnLine Database
- The Genome News Network
- NCBI Entrez Genome Project database
- NCBI Genome Primer
- GeneCards—an integrated database of human genes
- BBC News – Final genome 'chapter' published
- IMG (The Integrated Microbial Genomes system)—for genome analysis by the DOE-JGI
- GeKnome Technologies Next-Gen Sequencing Data Analysis—next-generation sequencing data analysis for Illumina and 454 Service from GeKnome Technologies (archived 3 March 2012)
ur:موراثہ
 |
