Biology:ATAC-seq
ATAC-seq (Assay for Transposase-Accessible Chromatin using sequencing) is a technique used in molecular biology to assess genome-wide chromatin accessibility.[1] In 2013, the technique was first described as an alternative advanced method for MNase-seq, FAIRE-Seq and DNase-Seq.[1] ATAC-seq is a faster analysis of the epigenome than DNase-seq or MNase-seq.[2][3][4]
Description
ATAC-seq identifies accessible DNA regions by probing open chromatin with hyperactive mutant Tn5 Transposase that inserts sequencing adapters into open regions of the genome.[2][5] While naturally occurring transposases have a low level of activity, ATAC-seq employs the mutated hyperactive transposase.[6] In a process called "tagmentation", Tn5 transposase cleaves and tags double-stranded DNA with sequencing adaptors.[7][8] The tagged DNA fragments are then purified, PCR-amplified, and sequenced using next-generation sequencing.[8] Sequencing reads can then be used to infer regions of increased accessibility as well as to map regions of transcription factor binding sites and nucleosome positions.[2] The number of reads for a region correlate with how open that chromatin is, at single nucleotide resolution.[2] ATAC-seq requires no sonication or phenol-chloroform extraction like FAIRE-seq;[9] no antibodies like ChIP-seq;[10] and no sensitive enzymatic digestion like MNase-seq or DNase-seq.[11] ATAC-seq preparation can be completed in under three hours.[12]
Applications
ATAC-Seq analysis is used to investigate a number of chromatin-accessibility signatures. The most common use is nucleosome mapping experiments,[3] but it can be applied to mapping transcription factor binding sites,[13] adapted to map DNA methylation sites,[14] or combined with sequencing techniques.[15]
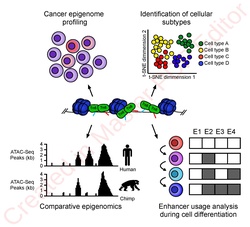
The utility of high-resolution enhancer mapping ranges from studying the evolutionary divergence of enhancer usage (e.g. between chimps and humans) during development[16] and uncovering a lineage-specific enhancer map used during blood cell differentiation.[17]
ATAC-Seq has also been applied to defining the genome-wide chromatin accessibility landscape in human cancers,[18] and revealing an overall decrease in chromatin accessibility in macular degeneration.[19] Computational footprinting methods can be performed on ATAC-seq to find cell specific binding sites and transcription factors with cell specific activity.[13]
Single-cell ATAC-seq
Modifications to the ATAC-seq protocol have been made to accommodate single-cell analysis. Microfluidics can be used to separate single nuclei and perform ATAC-seq reactions individually.[12] With this approach, single cells are captured by either a microfluidic device or a liquid deposition system before tagmentation.[12][20] An alternative technique that does not require single cell isolation is combinatorial cellular indexing.[21] This technique uses barcoding to measure chromatin accessibility in thousands of individual cells; it can generate epigenomic profiles from 10,000-100,000 cells per experiment.[22] But combinatorial cellular indexing requires additional, custom-engineered equipment or a large quantity of custom, modified Tn5.[23] Recently, a pooled barcode method called sci-CAR was developed, allowing joint profiling of chromatin accessibility and gene expression of single cells.[24]
Computational analysis of scATAC-seq is based on construction of a count matrix with number of reads per open chromatin regions. Open chromatin regions can be defined, for example, by standard peak calling of pseudo bulk ATAC-seq data. Further steps include data reduction with PCA and clustering of cells.[20] scATAC-seq matrices can be extremely large (hundreds of thousands of regions) and is extremely sparse, i.e. less than 3% of entries are non-zero.[25] Therefore, imputation of count matrix is another crucial step by using methods as non-negative matrix factorization. As with bulk ATAC-seq, scATAC-seq allows finding regulators like transcription factors controlling gene expression of cells. This can be achieved by looking at the number of reads around TF motifs[26] or footprinting analysis.[25]
References
- ↑ 1.0 1.1 "Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position". Nature Methods 10 (12): 1213–8. December 2013. doi:10.1038/nmeth.2688. PMID 24097267.
- ↑ 2.0 2.1 2.2 2.3 "ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide". Current Protocols in Molecular Biology 109: 21.29.1–21.29.9. January 2015. doi:10.1002/0471142727.mb2129s109. PMID 25559105.
- ↑ 3.0 3.1 "Structured nucleosome fingerprints enable high-resolution mapping of chromatin architecture within regulatory regions". Genome Research 25 (11): 1757–70. November 2015. doi:10.1101/gr.192294.115. PMID 26314830.
- ↑ "DNase-seq: a high-resolution technique for mapping active gene regulatory elements across the genome from mammalian cells". Cold Spring Harbor Protocols 2010 (2): pdb.prot5384. February 2010. doi:10.1101/pdb.prot5384. PMID 20150147.
- ↑ Bajic, Marko; Maher, Kelsey A.; Deal, Roger B. (2018). "Identification of Open Chromatin Regions in Plant Genomes Using ATAC-Seq". Plant Chromatin Dynamics. Methods in Molecular Biology. 1675. pp. 183–201. doi:10.1007/978-1-4939-7318-7_12. ISBN 978-1-4939-7317-0.
- ↑ "Transposon Tn5". Annual Review of Genetics 42 (1): 269–86. 2008. doi:10.1146/annurev.genet.42.110807.091656. PMID 18680433.
- ↑ Adey, Andrew (December 2010). "Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition". Genome Biology 11 (12): R119. doi:10.1186/gb-2010-11-12-r119. PMID 21143862.
- ↑ 8.0 8.1 "Tn5 transposase and tagmentation procedures for massively scaled sequencing projects". Genome Research 24 (12): 2033–40. December 2014. doi:10.1101/gr.177881.114. PMID 25079858.
- ↑ "Using formaldehyde-assisted isolation of regulatory elements (FAIRE) to isolate active regulatory DNA". Nature Protocols 7 (2): 256–67. January 2012. doi:10.1038/nprot.2011.444. PMID 22262007.
- ↑ "CETCh-seq: CRISPR epitope tagging ChIP-seq of DNA-binding proteins". Genome Research 25 (10): 1581–9. October 2015. doi:10.1101/gr.193540.115. PMID 26355004.
- ↑ Hoeijmakers, Wieteke Anna Maria; Bártfai, Richárd (2018). "Characterization of the Nucleosome Landscape by Micrococcal Nuclease-Sequencing (MNase-seq)". Chromatin Immunoprecipitation. Methods in Molecular Biology. 1689. pp. 83–101. doi:10.1007/978-1-4939-7380-4_8. ISBN 978-1-4939-7379-8.
- ↑ 12.0 12.1 12.2 "Single-cell chromatin accessibility reveals principles of regulatory variation". Nature 523 (7561): 486–90. July 2015. doi:10.1038/nature14590. PMID 26083756. Bibcode: 2015Natur.523..486B.
- ↑ 13.0 13.1 Li, Zhijian; Schulz, Marcel H.; Look, Thomas; Begemann, Matthias; Zenke, Martin; Costa, Ivan G. (26 February 2019). "Identification of transcription factor binding sites using ATAC-seq". Genome Biology 20 (1): 45. doi:10.1186/s13059-019-1642-2. PMID 30808370.
- ↑ "methyl-ATAC-seq measures DNA methylation at accessible chromatin". Genome Research 29 (6): 969–977. June 2019. doi:10.1101/gr.245399.118. PMID 31160376.
- ↑ Hendrickson, David G.; Soifer, Ilya; Wranik, Bernd J.; Botstein, David; Scott McIsaac, R. (2018), "Simultaneous Profiling of DNA Accessibility and Gene Expression Dynamics with ATAC-Seq and RNA-Seq", Computational Cell Biology, Methods in Molecular Biology, 1819, Springer New York, pp. 317–333, doi:10.1007/978-1-4939-8618-7_15, ISBN 9781493986170, PMID 30421411
- ↑ "Enhancer divergence and cis-regulatory evolution in the human and chimp neural crest". Cell 163 (1): 68–83. September 2015. doi:10.1016/j.cell.2015.08.036. PMID 26365491.
- ↑ "Immunogenetics. Chromatin state dynamics during blood formation". Science 345 (6199): 943–9. August 2014. doi:10.1126/science.1256271. PMID 25103404.
- ↑ "The chromatin accessibility landscape of primary human cancers". Science 362 (6413): eaav1898. October 2018. doi:10.1126/science.aav1898. PMID 30361341. Bibcode: 2018Sci...362.1898C.
- ↑ "ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration". Nature Communications 9 (1): 1364. April 2018. doi:10.1038/s41467-018-03856-y. PMID 29636475. Bibcode: 2018NatCo...9.1364W.
- ↑ 20.0 20.1 "High-throughput chromatin accessibility profiling at single-cell resolution". Nature Communications 9 (1): 3647. September 2018. doi:10.1038/s41467-018-05887-x. PMID 30194434. Bibcode: 2018NatCo...9.3647M.
- ↑ Cusanovich, Darren (May 2015). "Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing". Science 348 (6237): 910–914. doi:10.1126/science.aab1601. PMID 25953818. Bibcode: 2015Sci...348..910C.
- ↑ "Droplet-based combinatorial indexing for massive scale single-cell epigenomics". bioRxiv. 2019. doi:10.1101/612713.
- ↑ "A rapid and robust method for single cell chromatin accessibility profiling". Nature Communications 9 (1): 5345. December 2018. doi:10.1038/s41467-018-07771-0. PMID 30559361. Bibcode: 2018NatCo...9.5345C.
- ↑ Cao, Junyue; Cusanovich, Darren A.; Ramani, Vijay; Aghamirzaie, Delasa; Pliner, Hannah A.; Hill, Andrew J.; Daza, Riza M.; McFaline-Figueroa, Jose L. et al. (2018-09-28). "Joint profiling of chromatin accessibility and gene expression in thousands of single cells" (in en). Science 361 (6409): 1380–1385. doi:10.1126/science.aau0730. ISSN 0036-8075. PMID 30166440. Bibcode: 2018Sci...361.1380C.
- ↑ 25.0 25.1 Li, Zhijian; Kuppe, Christoph; Cheng, Mingbo; Menzel, Sylvia; Zenke, Martin; Kramann, Rafael et al. (2021). "Chromatin-accessibility estimation from single-cell ATAC-seq data with scOpen" (in en). Nature Communications 12 (1): 865931. doi:10.1038/s41467-021-26530-2. PMID 34737275. Bibcode: 2021NatCo..12.6386L.
- ↑ "chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data". Nature Methods 14 (10): 975–978. October 2017. doi:10.1038/nmeth.4401. PMID 28825706.
External source
- ATAC-seq probes open-chromatin state (figure)
- ATAC-seq: Fast and sensitive epigenomic profiling
- HINT-ATAC: Identification of Transcription Factor Binding Sites using ATAC-seq
 |