Biology:Karenia brevis
| Karenia brevis | |
|---|---|
| |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | SAR |
| Clade: | Alveolata |
| Phylum: | Myzozoa |
| Superclass: | Dinoflagellata |
| Class: | Dinophyceae |
| Order: | Gymnodiniales |
| Family: | Kareniaceae |
| Genus: | Karenia |
| Species: | K. brevis
|
| Binomial name | |
| Karenia brevis (Davis) G. Hansen et Moestrup
| |
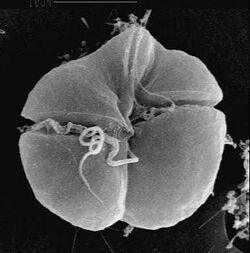
Karenia brevis is a microscopic, single-celled, photosynthetic organism in the genus Karenia. It is a marine dinoflagellate commonly found in the waters of the Gulf of Mexico.[1] It is the organism responsible for the "Florida red tides" that affect the Gulf coasts of Florida and Texas in the U.S., and nearby coasts of Mexico. K. brevis has been known to travel great lengths around the Florida peninsula and as far north as the Carolinas.[2]
Each cell has two flagella that allow it to move through the water in a spinning motion. K. brevis is unarmored, and does not contain peridinin. Cells are between 20 and 40 μm in diameter. K. brevis naturally produces a suite of potent neurotoxins collectively called brevetoxins, which cause gastrointestinal and neurological problems in other organisms and are responsible for large die-offs of marine organisms and seabirds.[3]
History
The classification of K. brevis has changed over time as advances in technology are made.[4]
Karenia brevis was named for Dr. Karen A. Steidinger[5] in 2001, and was previously known as Gymnodinium breve and Ptychodiscus brevis. It was first named Gymnodinium brevis in 1948, but was later changed to Gymnodinium breve, which correlates with the guidelines of the International Code of Botanical Nomenclature. In 1979 it was categorized under the genus Ptychodiscus and named Ptychodiscus brevis as new research showed it fit better under this genus because of its morphology, biochemistry, and ultrastructure. Then in 1989, scientists agreed this organism should be referred to as its original name (G. breve). It was then reclassified and transferred to the new genus Karenia, which was established at the University of Copenhagen in 2000.
Karenia brevis was first identified in Florida in 1947, but anecdotal reports in the Gulf of Mexico date back to the 1530s.[1][6] Outbreaks of K. brevis have been known to occur since the Spanish explorers of the 15th and 16th centuries, as documented by Spanish explorers like Cabeza de Vaca.[citation needed] These explorers noted large fish kills that resemble the die offs seen in present-day due to K. brevis. C.C. Davis confirmed these die offs were due to K. brevis in 1948.[7]
Ecology and distribution
Karenia brevis has an optimum temperature range of 22–28 °C (72–82 °F),[8] an optimum salinity range of 25-45 Practical Salinity Units (PSU),[9] has adapted to "low-irradiance environments," and can utilize both organic and inorganic nitrogen and phosphorus compounds to survive.[10] In its normal environment, K. brevis will move in the direction of greater light[11] and against the direction of gravity,[12] which will tend to keep the organism at the surface of whatever body of water it is suspended within. The swimming speed of K. brevis is about one metre per hour[13] and the organism can be found throughout the year in the waters of the Gulf of Mexico at concentrations of ≤ 1,000 cell per liter.[2]
Scientists have been unable to determine a definitive geographic range for K. brevis specifically because it is difficult to separate from the ten other species of Karenia, but K. brevis is the most common species occurring in the Gulf of Mexico.[14]
Karenia brevis is the causative agent of red tide, which occurs when the organism multiplies to higher than normal concentrations. During these events the water can take on a reddish or pinkish coloration, giving these explosions in the K. brevis population the name of Florida Red Tide. These algal blooms caused by K. brevis produce brevetoxins, which can result in significant ecological impacts through the death of large numbers of marine animals and birds, to include marine mammals.[15] Large scale fish kills are known to occur due to these Florida Red Tides caused by K. brevis. Fish species through the food chain are impacted, up to and including large predatory species such as sharks, as well as species typical in human consumption.[2]
One researcher has stated that, "There is no single hypothesis that can account for blooms of K. brevis along the west coast of Florida".[10] However, like most algae, their occurrence and survival depends on a variety of factors in their environment including water temperature, salinity, light, and nutrients/compounds present in the water.[10] However it is suspected that abundant use of fertilizers in surrounding coastal areas as well as fertilizer run-off from more distant farms, carried by the rivers, might have an impact on algae growth.
Under favorable conditions, toxin-producing dinoflagellates such as K. brevis flourish and grow to high concentrations, an event termed a "harmful algal bloom" or a "HAB". While there are many different types of these HABs and the effects can vary, K. brevis is the causative agent of Florida Red Tides. Due to the toxin that K. brevis produces, these red tides can be detrimental to marine life and can even affect human populations along coasts where they occur.[16]
Impact on human health and activities
In areas where K. brevis is found at normal population levels, the organism is not known to cause harm to human health. It is only at times of unchecked population growth, resulting in harmful algal blooms, when the organism is of concern to human health and activities.[15] The same cannot be said of shellfish harvested and consumed from these algal bloom areas. The brevetoxins released by K. brevis can be found in the flesh of shellfish during Florida Red Tides, potentially causing a condition known as Neurotoxic Shellfish Poisoning (NSP) in humans. Although no recorded human deaths have occurred from NSP, the poisoning does result in nausea, vomiting and a variety of neurological symptoms.[17] Other than NSP, the effects on human health during Florida Red Tide are thought to be limited to respiratory and eye irritation to susceptible persons on the water or close to the shore of areas impacted by the Red Tide, and irritation of skin directly exposed to Florida Red Tide waters. Persons with pre-existing respiratory conditions such as asthma, emphysema or COPD may be more susceptible to harm from the respiratory irritation caused by K. brevis and may be advised to remain away from coastal areas during periods of Florida Red Tide.[15]
The uncontrolled mass explosions of K. brevis populations resulting in Florida Red Tide also has a significant financial impact on the affected coastal areas. The primary source of revenue generation in many of the communities affected by K. brevis red tides is tourism. During periods of red tides this important source of revenue is often lost to the impacted coastal communities of Florida, often on the scale of tens of millions of dollars.[18]
This particular protist is known to be harmful to humans, large fish, and other marine mammals. It has been found that the survival of scleractinian coral is negatively affected by brevetoxin. Scleractinian coral exhibits decreased rates of respiration when there is a high concentration of K. brevis.[3]
Effect of Karenia brevis on Environment
Brevetoxins are a group of neurotoxic compounds released by K. brevis. At high concentration these brevetoxins can be fatal to fish, marine mammals, and birds.[19][20] Brevetoxis also pose a threat to corals.[21]
Large nearshore fish fatalities are caused by red-tide blooms.[22] Shorebirds can also get infected with brevetoxins by consuming fish.[23] Thus, red-tide blooms can have major level effects impacting the whole ecosystem. Additionally infected fish and shellfish pose a threat to the fishing industry and economy.[20][22]
K. brevis red tides have also been found to be a significant factor in the mortality of multiple species of sea turtles.[24] Specifically Kemp's ridleys, loggerheads, green turtles, and hawksbills, particularly along the west Florida coast.[24] Since red tide is a major cause of stranded sea turtles, it contributes to the vulnerability of this endangered species.[24]
K. brevis blooms pose other lethal health risks to marine animals like manatees. Extended occurrences of red tide blooms in the Gulf of Mexico have been associated with substantial instances of mortality in manatee populations[25]. Brevetoxins can lower manatee’s immune systems making them more at risk for other diseases.[25] Additionally, brevetoxin has been correlated with oxidative stress in manatees.[25]
Overall brevetoxins have grave effects on wildlife, and the multitude and compounded effects on entire marine ecosystems are not yet fully understood.
Detection and monitoring
Traditional methods for the detection of K. brevis are based on microscopy or pigment analysis. These are time-consuming, and typically require a skilled microscopist for identification.[26] Cultivation-based identification is extremely difficult and can take several months.
The traditional methods of detection and monitoring of K. brevis blooms from field measurements is labor-intensive and suffers from practical limitations on achieving real-time detection or monitoring. The "Brevebuster" is a deploy-able instrument that can be deployed on automated underwater vehicles or on stationary platforms that can optically detect the Florida red tides.[6] A molecular, real-time PCR-based approach for sensitive and accurate detection of K. brevis cells in marine environments has therefore been developed.[27] A real-time nucleic acid sequence-based amplification (NASBA) assay has been developed for detection of rbcL mRNA from K. brevis. NASBA is sensitive, rapid and effective, and may be used as an additional or alternative method to detect and quantify K. brevis in the marine environment.[28]
Another technique for the detection of K. brevis is multiwavelength spectroscopy, which uses a model-based examination of UV-vis spectra.[29] Methods of detection using satellite spectroscopy have also been developed.[30][31] Satellite images from Medium Resolution Imaging Spectrometer (MERIS) and Moderate Resolution Imaging Spectroradiometer (MODIS) ocean color sensor, identify K. brevis by making use of its chlorophyll fluorescence and low backscattering characteristics.[32][33][34] In addition to methods of detection of cells of K. brevis, enzyme-linked immunosorbent assay (ELISA) and liquid chromatography mass spectrometry (LCMS) have been developed for detecting brevetoxin in shellfish,[6][35] are more sensitive than the standard mouse bioassay, and as of 2008, were being considered by the Interstate Shellfish Sanitation Conference for regulatory use.
References
- ↑ 1.0 1.1 Magaña, Hugo A.; Contreras, Cindy; Villareal, Tracy A. (August 2003). "A historical assessment of Karenia brevis in the western Gulf of Mexico". Harmful Algae 2 (3): 163–171. doi:10.1016/s1568-9883(03)00026-x. ISSN 1568-9883.
- ↑ 2.0 2.1 2.2 "About Florida Red Tides". http://myfwc.com/research/redtide/general/about/.
- ↑ 3.0 3.1 Ross, Cliff; Ritson-Williams, Raphael; Pierce, Richard; Bullington, J. Bradley; Henry, Michael; Paul, Valerie J. (February 2010). "Effects of the Florida Red Tide Dinoflagellate, Karenia brevis, on Oxidative Stress and Metamorphosis of Larvae of the Coral Porites astreoides". Harmful Algae 9 (2): 173–9. doi:10.1016/j.hal.2009.09.001.
- ↑ "Red Tide K. Reikowski BIO 203". http://bioweb.uwlax.edu/bio203/s2013/reikowsk_kyli/classification.htm.
- ↑ "Bay Soundings". http://baysoundings.com/legacy-archives/sum03/profile.html.
- ↑ 6.0 6.1 6.2 Lopez CB, Dortch Q, Jewett EB, Garrison D (2008). Scientific assessment of marine harmful algal blooms. Interagency Working Group on Harmful Algal Blooms, Hypoxia, and Human Health of the Joint Subcommittee on Ocean Science and Technology. Washington, D.C.
- ↑ Brand, Larry E.; Compton, Angela (February 2007). "Long-term increase in Karenia brevis abundance along the Southwest Florida Coast". Harmful Algae 6 (2): 232–252. doi:10.1016/j.hal.2006.08.005. ISSN 1568-9883. PMID 18437245.
- ↑ Steidinger, K. A.; Ingle, R. M. (January 1972). "Observations on the 1971 Summer Red Tide in Tampa Bay, Florida". Environmental Letters 3 (4): 271–278. doi:10.1080/00139307209435473. ISSN 0013-9300. PMID 4627989.
- ↑ Magana, Hugo; Villareal, Tracy (1 March 2006). "The effect of environmental factors on the growth rate of Karenia brevis (Davis) G. Hansen and Moestrup". Harmful Algae 5 (2): 192–198. doi:10.1016/j.hal.2005.07.003. https://www.researchgate.net/publication/228492238.
- ↑ 10.0 10.1 10.2 Vargo, Gabriel A. (1 March 2009). "A brief summary of the physiology and ecology of Karenia brevis Davis (G. Hansen and Moestrup comb. nov.) red tides on the West Florida Shelf and of hypotheses posed for their initiation, growth, maintenance, and termination". Harmful Algae 8 (4): 573–584. doi:10.1016/j.hal.2008.11.002. ISSN 1568-9883.
- ↑ Geesey, M. E., and P. A. Tester. 1993. Gymnodinium breveGymnodinium breve: ubiquitous in Gulf of Mexico waters, p. 251-256. InIn T. J. S. Smayda and Shimizu (ed.), Toxic phytoplankton blooms in the sea: Proceedings of the Fifth International Conference on Toxic Marine Phytoplankton. Elsevier Science Publishing, Inc., New York, N.Y.
- ↑ Kamykowski, D.; Milligan, E. J.; Reed, R. E. (1998). "Relationships between geotaxis/phototaxis and diel vertical migration in autotrophic dinoflagellates". J. Plankton Res. 20 (9): 1781–1796. doi:10.1093/plankt/20.9.1781.
- ↑ Steidinger, K. A.; Joyce Jr, E. A. (1973). "Florida red tides". State Fla. Dep. Nat. Resour. Educat. Ser. 17: 1–26.
- ↑ Haywood, Allison J.; Steidinger, Karen A.; Truby, Earnest W.; Bergquist, Patricia R.; Bergquist, Peter L.; Adamson, Janet; Mackenzie, Lincoln (23 January 2004). "Comparative Morphology and Molecular Phylogenetic Analysis of Three New Species of the Genus Karenia (Dinophyceae) from New Zealand1". Journal of Phycology 40 (1): 165–179. doi:10.1111/j.0022-3646.2004.02-149.x. ISSN 0022-3646. Bibcode: 2004JPcgy..40..165H.
- ↑ 15.0 15.1 15.2 Hoagland, Porter; Jin, Di; Beet, Andrew; Kirkpatrick, Barbara; Reich, Andrew; Ullmann, Steve; Fleming, Lora E.; Kirkpatrick, Gary (July 2014). "The human health effects of Florida Red Tide (FRT) blooms: An expanded analysis". Environment International 68: 144–153. doi:10.1016/j.envint.2014.03.016. ISSN 0160-4120. PMID 24727069.
- ↑ Anderson, Donald M. (August 1997). "Turning back the harmful red tide". Nature 388 (6642): 513–514. doi:10.1038/41415. ISSN 0028-0836. Bibcode: 1997Natur.388..513A.
- ↑ Reich, Andrew; Lazensky, Rebecca; Faris, Jeremy; Fleming, Lora E.; Kirkpatrick, Barbara; Watkins, Sharon; Ullmann, Steve; Kohler, Kate et al. (March 2015). "Assessing the impact of shellfish harvesting area closures on neurotoxic shellfish poisoning (NSP) incidence during red tide (Karenia brevis) blooms". Harmful Algae 43: 13–19. doi:10.1016/j.hal.2014.12.003. ISSN 1568-9883.
- ↑ Larkin, Sherry L.; Adams, Charles M. (27 August 2007). "Harmful Algal Blooms and Coastal Business: Economic Consequences in Florida". Society & Natural Resources 20 (9): 849–859. doi:10.1080/08941920601171683. ISSN 0894-1920. Bibcode: 2007SNatR..20..849L.
- ↑ Hoagland, Porter; Jin, Di; Polansky, Lara Y.; Kirkpatrick, Barbara; Kirkpatrick, Gary; Fleming, Lora E.; Reich, Andrew; Watkins, Sharon M. et al. (August 2009). "The Costs of Respiratory Illnesses Arising from Florida Gulf Coast Karenia brevis Blooms" (in en). Environmental Health Perspectives 117 (8): 1239–1243. doi:10.1289/ehp.0900645. ISSN 0091-6765. PMID 19672403.
- ↑ 20.0 20.1 Vilas, Daniel; Buszowski, Joe; Sagarese, Skyler; Steenbeek, Jeroen; Siders, Zach; Chagaris, David (2023-02-13). "Evaluating red tide effects on the West Florida Shelf using a spatiotemporal ecosystem modeling framework" (in en). Scientific Reports 13 (1): 2541. doi:10.1038/s41598-023-29327-z. ISSN 2045-2322. PMID 36781942.
- ↑ Reynolds, David A.; Yoo, Mi-Jeong; Dixson, Danielle L.; Ross, Cliff (2020-02-07). "Exposure to the Florida red tide dinoflagellate, Karenia brevis, and its associated brevetoxins induces ecophysiological and proteomic alterations in Porites astreoides" (in en). PLOS ONE 15 (2): e0228414. doi:10.1371/journal.pone.0228414. ISSN 1932-6203. PMID 32032360. Bibcode: 2020PLoSO..1528414R.
- ↑ 22.0 22.1 Gannon, Damon P.; McCabe, Elizabeth J. Berens; Camilleri, Sandra A.; Gannon, Janet G.; Brueggen, Mary K.; Barleycorn, Aaron A.; Palubok, Valeriy I.; Kirkpatrick, Gary J. et al. (2009-03-12). "Effects of Karenia brevis harmful algal blooms on nearshore fish communities in southwest Florida" (in en). Marine Ecology Progress Series 378: 171–186. doi:10.3354/meps07853. ISSN 0171-8630. Bibcode: 2009MEPS..378..171G. https://www.int-res.com/abstracts/meps/v378/p171-186/.
- ↑ Deventer, Michelle van; Atwood, Karen; Vargo, Gabriel A.; Flewelling, Leanne J.; Landsberg, Jan H.; Naar, Jerome P.; Stanek, Danielle (2012-02-01). "Karenia brevis red tides and brevetoxin-contaminated fish: a high risk factor for Florida's scavenging shorebirds?" (in en). Botanica Marina 55 (1): 31–37. doi:10.1515/bot.2011.122. ISSN 1437-4323. https://www.degruyter.com/document/doi/10.1515/bot.2011.122/html.
- ↑ 24.0 24.1 24.2 Foley, Allen M.; Stacy, Brian A.; Schueller, Paul; Flewelling, Leanne J.; Schroeder, Barbara; Minch, Karrie; Fauquier, Deborah A.; Foote, Jerris J. et al. (2019-01-10). "Assessing Karenia brevis red tide as a mortality factor of sea turtles in Florida, USA" (in en). Diseases of Aquatic Organisms 132 (2): 109–124. doi:10.3354/dao03308. ISSN 0177-5103. PMID 30628577. https://www.int-res.com/abstracts/dao/v132/n2/p109-124/.
- ↑ 25.0 25.1 25.2 Walsh, Catherine J.; Butawan, Matthew; Yordy, Jennifer; Ball, Ray; Flewelling, Leanne; de Wit, Martine; Bonde, Robert K. (2015-04-01). "Sublethal red tide toxin exposure in free-ranging manatees (Trichechus manatus) affects the immune system through reduced lymphocyte proliferation responses, inflammation, and oxidative stress". Aquatic Toxicology 161: 73–84. doi:10.1016/j.aquatox.2015.01.019. ISSN 0166-445X. PMID 25678466. https://www.sciencedirect.com/science/article/pii/S0166445X15000272.
- ↑ Millie, D. F.; Schofield, O. M.; Kirkpatrick, G. J.; Hohnsen, G.; Tester, P. A.; Vinyard, B. T. (1997). "Detection of harmful algal blooms using photopigments and absorption signatures: a case study of the Florida red tide dinoflagellate, Gymnodinium breve. Gymnodinium breve". Limnol. Oceanogr. 42 (5part2): 1240–1251. doi:10.4319/lo.1997.42.5_part_2.1240. https://digitalcommons.odu.edu/cgi/viewcontent.cgi?article=1359&context=oeas_fac_pubs.
- ↑ Gray, M.; B. Wawrik; E. Caspar; J.H. Paul (2003). "Molecular Detection and Quantification of the Red Tide Dinoflagellate Karenia brevis in the Marine Environment". Applied and Environmental Microbiology 69 (9): 5726–5730. doi:10.1128/AEM.69.9.5726-5730.2003. PMID 12957971. Bibcode: 2003ApEnM..69.5726G.
- ↑ Casper, Erica T.; Paul, John H.; Smith, Matthew C.; Gray, Michael (1 August 2004). "Detection and Quantification of the Red Tide Dinoflagellate Karenia brevis by Real-Time Nucleic Acid Sequence-Based Amplification". Appl. Environ. Microbiol. 70 (8): 4727–4732. doi:10.1128/AEM.70.8.4727-4732.2004. ISSN 0099-2240. PMID 15294808. Bibcode: 2004ApEnM..70.4727C.
- ↑ Spear, H. Adam, K. Daly, D. Huffman, and L. Garcia-Rubio. 2009. Progress in developing a new detection method for the harmful algal bloom species, Karenia brevis, through multiwavelength spectroscopy. HARMFUL ALGAE. 8:189–195.
- ↑ Hu, C., et al. (2005) Red tide detection and tracing using MODIS fluorescence data: A regional example in SW Florida coastal waters, Remote Sensing of Environment 97(2005) 311–321 http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.115.4645&rep=rep1&type=pdf
- ↑ Carvalho, G., et al. (2007) Detection of Florida "red tides" from SeaWiFS and MODIS imagery, Anais XIII Simposio Brasileiro de Sensoriamento Remoto, 21–26 Abril 2007 http://marte.dpi.inpe.br/col/dpi.inpe.br/sbsr@80/2006/11.07.00.35/doc/4581-4588.pdf
- ↑ Amin, Ruhul; Zhou, Jing; Gilerson, Alex; Gross, Barry; Moshary, Fred; Ahmed, Samir (25 May 2009). "Novel optical techniques for detecting and classifying toxic dinoflagellate Karenia brevis blooms using satellite imagery". Optics Express 17 (11): 9126–9144. doi:10.1364/OE.17.009126. ISSN 1094-4087. PMID 19466162. Bibcode: 2009OExpr..17.9126A.
- ↑ Cannizzaro, Jennifer P.; Hu, Chuanmin; English, David C.; Carder, Kendall L.; Heil, Cynthia A.; Müller-Karger, Frank E. (September 2009). "Detection of Karenia brevis blooms on the west Florida shelf using in situ backscattering and fluorescence data". Harmful Algae 8 (6): 898–909. doi:10.1016/j.hal.2009.05.001. ISSN 1568-9883.
- ↑ Soto, Inia M.; Cannizzaro, Jennifer; Muller-Karger, Frank E.; Hu, Chuanmin; Wolny, Jennifer; Goldgof, Dmitry (December 2015). "Evaluation and optimization of remote sensing techniques for detection of Karenia brevis blooms on the West Florida Shelf". Remote Sensing of Environment 170: 239–254. doi:10.1016/j.rse.2015.09.026. ISSN 0034-4257. Bibcode: 2015RSEnv.170..239S.
- ↑ Dickey, R. W.; Plakas, S. M.; Jester, E. L.; El Said, K. R.; Johannessen, J. N.; Flewelling, L. J.; Scott, P.; Hammond, D. G. et al. (2004). "Multi-Laboratory Study of Five Methods for the Determination of Brevetoxins in Shellfish Tissue Extracts". Harmful Algae 2002 : Proceedings of the Xth International Conference on Harmful Algae, St. Pete Beach, Florida, USA, October 21-25, 2002. International Conference on Harmful Algae (10th : 2002 : St. Pete Beach, Florida). 10. pp. 300–302.
Glibert, P.M.; Burkholder, J.M (22 May 2014). "The Complex Relationships Between Increases in Fertilization of the Earth, Coastal Eutrophication and Proliferation of Harmful Algal Blooms". Ecology of Harmful Algae. Ecological Studies. 189. pp. 341–354. doi:10.1007/978-3-540-32210-8_26. ISBN 978-3-540-32209-2.
Further reading
- Campbell, Lisa; Pepper, Alan E.; Ryan, Darcie E. (11 October 2014). "De novo assembly and characterization of the transcriptome of the toxic dinoflagellate Karenia brevis". BMC Genomics 15 (888): 888. doi:10.1186/1471-2164-15-888. PMID 25306556.
- Ryan, Darcie; Pepper, Alan; Campbell, Lisa (11 October 2014). "De novo assembly and characterization of the transcriptome of the toxic dinoflagellate Karenia brevis". BMC Genomics 15 (1): 888. doi:10.1186/1471-2164-15-888. PMID 25306556.
- Kirkpatrick, Barbara; Kohler, Kate; Byrne, Margaret (15 September 2014). "Human responses to Florida red tides: Policy awareness and adherence to local fertilizer ordinances". Science of the Total Environment 493: 898–909. doi:10.1016/j.scitotenv.2014.06.083. PMID 25003583. Bibcode: 2014ScTEn.493..898K.
- Özhan, Koray; Bargu, Sibel (10 June 2014). "Responses of sympatric Karenia brevis, Prorocentrum minimum, and Heterosigma akashiwo to the exposure of crude oil". Ecotoxicology 23 (8): 1387–1398. doi:10.1007/s10646-014-1281-z. PMID 25009015. Bibcode: 2014Ecotx..23.1387O.
- Naar, Jerome; Bourdelais, Andrea; Tomas, Carmelo (February 2002). "A Competitive ELISA to Detect Brevetoxins from Karenia brevis (Formerly Gymnodinium breve) in Seawater, Shellfish, and Mammalian Body Fluid". Environmental Health Perspectives 110 (2): 179–185. doi:10.1289/ehp.02110179. PMID 11836147.
- Meyer, Kevin A.; O' Neil, Judith M.; Hitchcock, Gary L.; Heil, Cynthia A. (September 2014). "Microbial production along the West Florida Shelf: Responses of bacteria and viruses to the presence and phase of Karenia brevis blooms". Harmful Algae 38 (Sp. Iss): 110–8. doi:10.1016/j.hal.2014.04.015.
External links
Wikidata ☰ Q293143 entry
 |


