Biology:LCCL domain
| LCCL | |||||||||
|---|---|---|---|---|---|---|---|---|---|
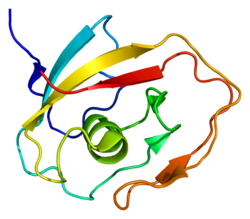 | |||||||||
| Identifiers | |||||||||
| Symbol | LCCL | ||||||||
| Pfam | PF03815 | ||||||||
| Pfam clan | CL0513 | ||||||||
| InterPro | IPR004043 | ||||||||
| CATH | 1jbi | ||||||||
| SCOP2 | 1jbi / SCOPe / SUPFAM | ||||||||
| |||||||||
In molecular biology, the LCCL domain is a protein domain which has been named after several well-characterised proteins that were found to contain it, namely Limulus clotting factor C, Cochlin (Coch-5b2) and Lgl1 (CRISPLD2). It is an about 100 amino acids domain whose C-terminal part contains a highly conserved histidine in a conserved motif YxxxSxxCxAAVHxGVI. The LCCL module is thought to be an autonomously folding domain that has been used for the construction of various modular proteins through exon-shuffling. It has been found in various metazoan proteins in association with complement B-type domains, C-type lectin domains, von Willebrand type A domains, CUB domains, discoidin lectin domains or CAP domains. It has been proposed that the LCCL domain could be involved in lipopolysaccharide (LPS) binding.[1][2] LCCL exhibits a novel fold.[3]
Some proteins known to contain a LCCL domain include Limulus factor C, an LPS endotoxin-sensitive trypsin type serine protease which serves to protect the organism from bacterial infection; vertebrate cochlear protein cochlin or coch-5b2 (Cochlin is probably a secreted protein, mutations affecting the LCCL domain of coch-5b2 cause the deafness disorder DFNA9 in humans); and mammalian late gestation lung protein Lgl1, contains two tandem copies of the LCCL domain.[4]
References
- ↑ "The LCCL module". Eur. J. Biochem. 267 (18): 5751–7. September 2000. doi:10.1046/j.1432-1327.2000.01641.x. PMID 10971586.
- ↑ "Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction". Nat. Genet. 20 (3): 299–303. November 1998. doi:10.1038/3118. PMID 9806553.
- ↑ Liepinsh, E; Trexler, M; Kaikkonen, A; Weigelt, J; Bányai, L; Patthy, L; Otting, G (1 October 2001). "NMR structure of the LCCL domain and implications for DFNA9 deafness disorder.". The EMBO Journal 20 (19): 5347–53. doi:10.1093/emboj/20.19.5347. PMID 11574466.
- ↑ "A novel developmentally regulated gene in lung mesenchyme: homology to a tumor-derived trypsin inhibitor". Am. J. Physiol. 276 (6 Pt 1): L1027-36. June 1999. doi:10.1152/ajplung.1999.276.6.L1027. PMID 10362728.
 |

