Chemistry:Rhodanine
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Sulfanylidene-1,3-thiazolidin-4-one | |
| Other names
2-Thioxo-4-thiazolidinone; 4-Oxo-2-thioxothiazoline
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H3NOS2 | |
| Molar mass | 133.18 g·mol−1 |
| Density | 0.868 g/cm−3[2] |
| Melting point | 170 °C (338 °F; 443 K) |
| Soluble[2] | |
| Solubility | Ethanol, dimethyl sulfoxide[2] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H318 | |
| P264, P270, P280, P301+312, P305+351+338, P310, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
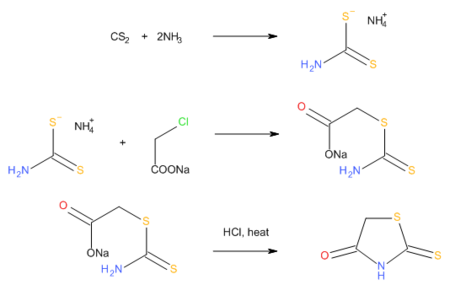
Rhodanine is a 5-membered heterocyclic organic compound possessing a thiazolidine core. It was discovered in 1877 by Marceli Nencki who named it "Rhodaninsaure" in reference to its synthesis from ammonium rhodanide (known as ammonium thiocyanate to modern chemists) and chloroacetic acid in water.[3]
Rhodanines can also be prepared by the reaction of carbon disulfide, ammonia, and chloroacetic acid, which proceeds via an intermediate dithiocarbamate.[4]
Derivatives
Some rhodanine derivatives have pharmacological properties; for instance, epalrestat is used to treat diabetic neuropathy. However, most are promiscuous binders with poor selectivity; as a result, this class of compounds is viewed with suspicion by medicinal chemists.[5][6][7] Differing academic opinions exist concerning the correct use of PAINS filters, the necessity of the experimental confirmations of such properties, and many useful features of rhodanine derivatives.[8][9]
References
- ↑ Rhodanine at Sigma-Aldrich
- ↑ 2.0 2.1 2.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedb1 - ↑ Nencki, M. (10 July 1877). "Ueber die Einwirkung der Monochloressigsäure auf Sulfocyansäure und ihre Salze". Journal für Praktische Chemie 16 (1): 1–17. doi:10.1002/prac.18770160101. https://zenodo.org/record/1427872.
- ↑ Redemann, C. Ernst; Icke, Roland N.; Alles, Gordon A. (1955). "Rhodanine". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv3p0763.; Collective Volume, 3, pp. 763
- ↑ Baell, J. B; Holloway, G. A (2010). "New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays". J. Med. Chem. 53 (7): 2719–2740. doi:10.1021/jm901137j. PMID 20131845.
- ↑ Tomašić, Tihomir; Peterlin Mašič, Lucija (2012). "Rhodanine as a scaffold in drug discovery: A critical review of its biological activities and mechanisms of target modulation". Expert Opinion on Drug Discovery 7 (7): 549–60. doi:10.1517/17460441.2012.688743. PMID 22607309.
- ↑ Pouliot, Martin; Jeanmart, Stephane (8 September 2015). "Pan Assay Interference Compounds (PAINS) and Other Promiscuous Compounds in Antifungal Research". Journal of Medicinal Chemistry 59 (2): 497–503. doi:10.1021/acs.jmedchem.5b00361. PMID 26313340.
- ↑ Kaminskyy, D; Kryshchyshyn, A; Lesyk, R (2017). "Recent developments with rhodanine as a scaffold for drug discovery". Expert Opinion on Drug Discovery 12 (12): 1233–1252. doi:10.1080/17460441.2017.1388370. PMID 29019278.
- ↑ Kaminskyy, D; Kryshchyshyn, A; Lesyk, R (2017). "5-Ene-4-thiazolidinonese An efficient tool in medicinal chemistry". Eur. J. Med. Chem. 140 (10): 542–594. doi:10.1016/j.ejmech.2017.09.031. PMID 28987611.
 |