Chemistry:Melflufen
| |
| Names | |
|---|---|
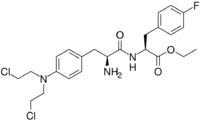
| IUPAC name
Ethyl (2S)-2-[[(2S)-2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoyl]amino]-3-(4-fluorophenyl)propanoate
| |
| Systematic IUPAC name
4-[Bis-(2-chloroethyl)amino]-L-phenylalanine-4-fluoro-L-phenylalanine ethyl ester | |
| Other names
J1
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C24H30Cl2FN3O3 | |
| Molar mass | 498.42 g·mol−1 |
| Pharmacology | |
| Pharmacokinetics: | |
| Aminopeptidase hydrolysis, Spontaneous hydrolyisis on N-mustard | |
| 10 min in vitro | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Melflufen (melphalan flufenamide, previously designated J1)[1][2] is an anticancer drug under development by Oncopeptides AB.[3] It is a peptidase enhanced cytotoxic (PEnC) that exerts a targeted delivery of melphalan in cells with high expression of aminopeptidases, such as aminopeptidase N, which has been described as over-expressed in human malignancies. Aminopeptidase N plays a functional role in malignant angiogenesis. Currently, melflufen is under evaluation in a multinational and randomized phase III study for the treatment of patients with relapsed and refractory multiple myeloma.[4]
Metabolism
Melflufen is metabolized by aminopeptidase hydrolysis and by spontaneous hydrolysis on N-mustard.[5] Its biological half-life is 10 minutes in vitro.
Origin and development
Melflufen is a peptidase enhanced cytotoxic (PEnC) with a targeted delivery within tumor cells of melphalan, a widely used classical chemotherapeutic belonging to a group of alkylating agents developed more than 50 years ago. Substantial clinical experience has been accumulated about melphalan since then. Numerous derivatives of melphalan, designed to increase the activity or selectivity, have been developed and investigated in vitro or in animal models.[6] Melflufen was synthesized, partly due to previous experience of an alkylating peptide cocktail named Peptichemio[7] and its anti-tumor activity is being investigated.
Pharmacology
Compared to melphalan, melflufen exhibits significantly higher in vitro and in vivo activity in several models of human cancer.[8][9][10][11][12][13][14][15] A preclinical study, performed at Dana–Farber Cancer Institute, demonstrated that melflufen induced apoptosis in multiple myeloma cell lines, even those resistant to conventional treatment (including melphalan).[16] In vivo effects in xenografted animals were also observed, and the results confirmed by M Chesi and co-workers – in a unique genetically engineered mouse model of multiple myeloma – are believed to be predictive of clinical efficacy.[17]
Structure
Chemically, the drug is best described as the ethyl ester of a dipeptide consisting of melphalan and the amino acid derivative para-fluoro-L-phenylalanine.
Pharmacokinetics
Pharmacokinetic analysis of plasma samples showed a rapid formation of melphalan; concentrations generally exceeded those of melflufen during ongoing infusion. Melflufen rapidly disappeared from plasma after infusion, while melphalan typically peaked a few minutes after the end of infusion. This suggests that melflufen is rapidly and widely distributed to extravasal tissues, in which melphalan is formed and thereafter redistributed to plasma.[18] This rapid disappearance from plasma is likely due to hydrolytic enzymes.[19] The Zn(2+) dependent ectopeptidase (also known as alanine aminopeptidase), degrades proteins and peptides with a N-terminal neutral amino acid. Aminopeptidase N is frequently overexpressed in tumors and has been associated with the growth of different human cancers suggesting it as a suitable target for anti-cancerous therapy.[20]
Adverse effects
In a human Phase 1 trial, no dose-limiting toxicities (DLTs) were observed at lower doses. At doses above 50 mg, reversible neutropenias and thrombocytopenias were observed, and particularly evident in heavily pretreated patients.[21] These side-effects are shared by most chemotherapies, including alkylating agents in general.
Drug interactions
No drug interaction studies have been reported. Several in vitro studies indicate that melflufen may be successfully combined with standard chemotherapy or targeted agents.[22][23]
Therapeutic efficacy
In a Phase 1/2 trial, in solid tumor patients refractory to standard therapy, response evaluation showed disease stabilization in a majority of patients.[24][25] In relapsed and refractory multiple-myeloma (RRMM) patients, promising activity was seen in heavily pre-treated RRMM patients where conventional therapies had failed; the median Progression-Free Survival was 9.4 months and the Duration of Response was 9.6 months.[26] An overall response rate of 41% and a clinical benefit rate of 56% were also shown, with similar results seen across patient populations regardless of their refractory status. Hematologic toxicity was common, but manageable with cycle prolongations, dose modifications and supportive therapy, and non-hematologic treatment-related adverse events were infrequent.
References
- ↑ Berglund, Åke; Ullén, Anders; Lisyanskaya, Alla; Orlov, Sergey; Hagberg, Hans; Tholander, Bengt; Lewensohn, Rolf; Nygren, Peter et al. (2015). "First-in-human, phase I/IIa clinical study of the peptidase potentiated alkylator melflufen administered every three weeks to patients with advanced solid tumor malignancies". Investigational New Drugs 33 (6): 1232–41. doi:10.1007/s10637-015-0299-2. PMID 26553306.
- ↑ Strese, Sara; Wickström, Malin; Fuchs, Peder Fredlund; Fryknäs, Mårten; Gerwins, Pär; Dale, Tim; Larsson, Rolf; Gullbo, Joachim (2013). "The novel alkylating prodrug melflufen (J1) inhibits angiogenesis in vitro and in vivo". Biochemical Pharmacology 86 (7): 888–95. doi:10.1016/j.bcp.2013.07.026. PMID 23933387.
- ↑ "Oncopeptides". https://oncopeptides.se/.
- ↑ https://clinicaltrials.gov/ct2/show/NCT01897714?term=melflufen&rank=1
- ↑ Gullbo, J; Tullberg, M; Våbenø, J; Ehrsson, H; Lewensohn, R; Nygren, P; Larsson, R; Luthman, K (2003). "Structure-activity relationship for alkylating dipeptide nitrogen mustard derivatives". Oncology Research 14 (3): 113–32. doi:10.3727/000000003771013071. PMID 14760861.
- ↑ Wickstrom, M.; Lovborg, H.; Gullbo, J. (2006). "Future Prospects for Old Chemotherapeutic Drugs in the Target-Specific Era; Pharmaceutics, Combinations, Co-Drugs and Prodrugs with Melphalan as an Example". Letters in Drug Design & Discovery 3 (10): 695. doi:10.2174/157018006778631893.
- ↑ Gullbo, J; Dhar, S; Luthman, K; Ehrsson, H; Lewensohn, R; Nygren, P; Larsson, R (2003). "Antitumor activity of the alkylating oligopeptides J1 (L-melphalanyl-p-L-fluorophenylalanine ethyl ester) and P2 (L-prolyl-m-L-sarcolysyl-p-L-fluorophenylalanine ethyl ester): Comparison with melphalan". Anti-Cancer Drugs 14 (8): 617–24. doi:10.1097/00001813-200309000-00006. PMID 14501383.
- ↑ Berglund, Åke; Ullén, Anders; Lisyanskaya, Alla; Orlov, Sergey; Hagberg, Hans; Tholander, Bengt; Lewensohn, Rolf; Nygren, Peter et al. (2015). "First-in-human, phase I/IIa clinical study of the peptidase potentiated alkylator melflufen administered every three weeks to patients with advanced solid tumor malignancies". Investigational New Drugs 33 (6): 1232–41. doi:10.1007/s10637-015-0299-2. PMID 26553306.
- ↑ Strese, Sara; Wickström, Malin; Fuchs, Peder Fredlund; Fryknäs, Mårten; Gerwins, Pär; Dale, Tim; Larsson, Rolf; Gullbo, Joachim (2013). "The novel alkylating prodrug melflufen (J1) inhibits angiogenesis in vitro and in vivo". Biochemical Pharmacology 86 (7): 888–95. doi:10.1016/j.bcp.2013.07.026. PMID 23933387.
- ↑ Wickström, M; Johnsen, J. I.; Ponthan, F; Segerström, L; Sveinbjörnsson, B; Lindskog, M; Lövborg, H; Viktorsson, K et al. (2007). "The novel melphalan prodrug J1 inhibits neuroblastoma growth in vitro and in vivo". Molecular Cancer Therapeutics 6 (9): 2409–17. doi:10.1158/1535-7163.MCT-07-0156. PMID 17876040.
- ↑ Gullbo, J; Lindhagen, E; Bashir-Hassan, S; Tullberg, M; Ehrsson, H; Lewensohn, R; Nygren, P; de la Torre, M et al. (2004). "Antitumor efficacy and acute toxicity of the novel dipeptide melphalanyl-p-L-fluorophenylalanine ethyl ester (J1) in vivo". Investigational New Drugs 22 (4): 411–20. doi:10.1023/B:DRUG.0000036683.10945.bb. PMID 15292711.
- ↑ Gullbo, J; Wickström, M; Tullberg, M; Ehrsson, H; Lewensohn, R; Nygren, P; Luthman, K; Larsson, R (2003). "Activity of hydrolytic enzymes in tumour cells is a determinant for anti-tumour efficacy of the melphalan containing prodrug J1". Journal of Drug Targeting 11 (6): 355–63. doi:10.1080/10611860310001647140. PMID 14668056.
- ↑ Gullbo, J; Dhar, S; Luthman, K; Ehrsson, H; Lewensohn, R; Nygren, P; Larsson, R (2003). "Antitumor activity of the alkylating oligopeptides J1 (L-melphalanyl-p-L-fluorophenylalanine ethyl ester) and P2 (L-prolyl-m-L-sarcolysyl-p-L-fluorophenylalanine ethyl ester): Comparison with melphalan". Anti-Cancer Drugs 14 (8): 617–24. doi:10.1097/00001813-200309000-00006. PMID 14501383.
- ↑ Chauhan, D.; Ray, A.; Viktorsson, K.; Spira, J.; Paba-Prada, C.; Munshi, N.; Richardson, P.; Lewensohn, R. et al. (2013). "In Vitro and in Vivo Antitumor Activity of a Novel Alkylating Agent, Melphalan-Flufenamide, against Multiple Myeloma Cells". Clinical Cancer Research 19 (11): 3019–31. doi:10.1158/1078-0432.CCR-12-3752. PMID 23584492.
- ↑ Viktorsson, K; Shah, C. H.; Juntti, T; Hååg, P; Zielinska-Chomej, K; Sierakowiak, A; Holmsten, K; Tu, J et al. (2016). "Melphalan-flufenamide is cytotoxic and potentiates treatment with chemotherapy and the Src inhibitor dasatinib in urothelial carcinoma". Molecular Oncology 10 (5): 719–34. doi:10.1016/j.molonc.2015.12.013. PMID 26827254.
- ↑ Chauhan, D; Ray, A; Viktorsson, K; Spira, J; Paba-Prada, C; Munshi, N; Richardson, P; Lewensohn, R et al. (2013). "In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells". Clinical Cancer Research 19 (11): 3019–31. doi:10.1158/1078-0432.CCR-12-3752. PMID 23584492.
- ↑ Chesi, M; Matthews, G. M.; Garbitt, V. M.; Palmer, S. E.; Shortt, J; Lefebure, M; Stewart, A. K.; Johnstone, R. W. et al. (2012). "Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy". Blood 120 (2): 376–85. doi:10.1182/blood-2012-02-412783. PMID 22451422.
- ↑ Berglund, Åke; Ullén, A; Lisyanskaya, A; Orlov, S; Hagberg, H; Tholander, B; Lewensohn, R; Nygren, P et al. (2015). "First-in-human, phase I/IIa clinical study of the peptidase potentiated alkylator melflufen administered every three weeks to patients with advanced solid tumor malignancies". Investigational New Drugs 33 (6): 1232–41. doi:10.1007/s10637-015-0299-2. PMID 26553306.
- ↑ Wickström, M; Viktorsson, K; Lundholm, L; Aesoy, R; Nygren, H; Sooman, L; Fryknäs, M; Vogel, L. K. et al. (2010). "The alkylating prodrug J1 can be activated by aminopeptidase N, leading to a possible target directed release of melphalan". Biochemical Pharmacology 79 (9): 1281–90. doi:10.1016/j.bcp.2009.12.022. PMID 20067771.
- ↑ Wickström, M; Larsson, R; Nygren, P; Gullbo, J (2011). "Aminopeptidase N (CD13) as a target for cancer chemotherapy". Cancer Science 102 (3): 501–8. doi:10.1111/j.1349-7006.2010.01826.x. PMID 21205077.
- ↑ Berglund, Åke; Ullén, A; Lisyanskaya, A; Orlov, S; Hagberg, H; Tholander, B; Lewensohn, R; Nygren, P et al. (2015). "First-in-human, phase I/IIa clinical study of the peptidase potentiated alkylator melflufen administered every three weeks to patients with advanced solid tumor malignancies". Investigational New Drugs 33 (6): 1232–41. doi:10.1007/s10637-015-0299-2. PMID 26553306.
- ↑ Wickström, M; Haglund, C; Lindman, H; Nygren, P; Larsson, R; Gullbo, J (2008). "The novel alkylating prodrug J1: Diagnosis directed activity profile ex vivo and combination analyses in vitro". Investigational New Drugs 26 (3): 195–204. doi:10.1007/s10637-007-9092-1. PMID 17922077.
- ↑ Chauhan, D; Ray, A; Viktorsson, K; Spira, J; Paba-Prada, C; Munshi, N; Richardson, P; Lewensohn, R et al. (2013). "In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells". Clinical Cancer Research 19 (11): 3019–31. doi:10.1158/1078-0432.CCR-12-3752. PMID 23584492.
- ↑ Berglund, Åke; Ullén, A; Lisyanskaya, A; Orlov, S; Hagberg, H; Tholander, B; Lewensohn, R; Nygren, P et al. (2015). "First-in-human, phase I/IIa clinical study of the peptidase potentiated alkylator melflufen administered every three weeks to patients with advanced solid tumor malignancies". Investigational New Drugs 33 (6): 1232–41. doi:10.1007/s10637-015-0299-2. PMID 26553306.
- ↑ Viktorsson, K; Shah, C. H.; Juntti, T; Hååg, P; Zielinska-Chomej, K; Sierakowiak, A; Holmsten, K; Tu, J et al. (2016). "Melphalan-flufenamide is cytotoxic and potentiates treatment with chemotherapy and the Src inhibitor dasatinib in urothelial carcinoma". Molecular Oncology 10 (5): 719–34. doi:10.1016/j.molonc.2015.12.013. PMID 26827254.
- ↑ https://ash.confex.com/ash/2015/webprogram/Paper85666.html

