Chemistry:Aminal
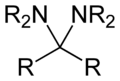
In organic chemistry, an aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: –C(NR
2)(NR
2)–. (As is customary in organic chemistry, R can represent hydrogen or an alkyl group).[1] A common aminal is bis(dimethylamino)methane, a colorless liquid that is prepared by the reaction of dimethylamine and formaldehyde:[2]
- 2 (CH
3)
2NH + CH
2O → [(CH
3)
2N]
2CH
2 + H
2O
Aminals are encountered in, for instance, the Fischer indole synthesis. Several examples exist in nature.[3]
- Naturally occurring aminals
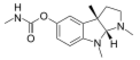
Physostigmine,a highly toxic cholinesterase inhibitor found in the Calabar bean.
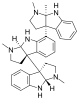
Hodgkinsine, an alkaloid with antiviral, antibacterial and antifungal effects
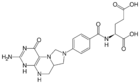
5,10-Methylenetetrahydrofolate, an intermediate in one-carbon metabolism
Hexahydro-1,3,5-triazine ((CH
2NH)
3), an intermediate in the condensation of formaldehyde and ammonia, tends to degrade to hexamethylene tetraamine.
Cyclic aminals can be obtained by the condensation of a diamine and an aldehyde.[4] Imidazolidines are one class of these cyclic aminals.
See also
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "aminals". doi:10.1351/goldbook.A00270
- ↑ Gaudry, Michel; Jasor, Yves; Khac, Trung Bui (1979). "Regioselective Mannich Condensation with Dimethyl(Methylene)ammonium Trifluoroacetate: 1-(Dimethylamino)-4-methyl-3-pentanone". Org. Synth. 59: 153. doi:10.15227/orgsyn.059.0153.
- ↑ Duhamel, Lucette (1982). "Aminals". in Saul Patai. Amino, Nitroso and Nitro Compounds and Their Derivatives: Vol. 2. PATAI'S Chemistry of Functional Groups. pp. 849–907. doi:10.1002/9780470771679.ch5. ISBN 9780470771679.
- ↑ Hiersemann, M. "Functions bearing two nitrogens" in Comprehensive Organic Functional Group Transformations II 2005, volume 4, 411-441. Edited by Katritzky, Alan R.; Taylor, Richard J. K. doi:10.1016/B0-08-044655-8/00075-1
 |