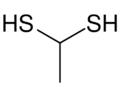
Chemistry:1,1-Ethanedithiol
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Ethane-1,1-dithiol | |||
| Other names
Methyldisulfanyl methane
1,1-Ethanedithiol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C2H6S2 | |||
| Molar mass | 94.19 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.83 g/cm3 | ||
| Boiling point | 71 °C (160 °F; 344 K) | ||
| 12.97 g/L | |||
| Solubility in water | Good solubility in most organic solvents | ||
Refractive index (nD)
|
1.37 | ||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H302, H312, H332 | |||
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P501 | |||
| Related compounds | |||
Related thiols
|
Ethanethiol; Methanedithiol; 1,2-Ethanedithiol; 1,1,1-Ethanetrithiol; 1-hydroxy-ethanethiol; propane-1,1-dithiol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1,1-Ethanedithiol is an organosulfur compound with formula CH3CH(SH)2. It is a colourless smelly liquid that is added to or found in some foods. The compound is an example of a geminal dithiol.
Use
Flavouring uses of ethane-1,1-dithiol may include drinks, oil, gravy, soup, meat, fruit, seasonings, and snacks. Maximum concentrations in use that are generally recognised as safe (GRAS) is five parts per million (ppm) but typical uses are around 0.2 ppm.[1] It is supplied as a 1% solution in ethanol, due to its strong offensive smell.[2] In the diluted form with 4% ethyl acetate and ethanol the CAS number is 69382-62-3.[3] Toxicity may be due to metabolism products hydrogen sulfide and acetaldehyde, however as used it has a margin of safety of over 10,000,000.[2] Other ways that it is modified in the body apart from hydrolysis is methylation to 1-methylsulfanyl-ethanethiol, oxidation of the sulfur to an ethyl sulfonate, glucuronidation of the sulfur, or combination with cysteine by way of a disulfide bridge.[2]
Identifiers
Since it is used in the food industry there are codes issued by various authorities. It is identified as JECFA number 1660.[4] The Flavor and Extract Manufacturers Association (FEMA) id is 4111.[1] The European designation for the flavouring is Fl 12.293.[5]
Natural occurrence
It can be produced during fermentation of grapes.[6] It is used as a food flavouring.[4]
It is found naturally in the scent of durian.[7][8]
Properties
The nuclear magnetic resonance spectrum shows three environments for protons, in the ratio of 3:2:1 corresponding to CH3 SH and CH.[9]
Reactions
1,1-Ethanedithiol has several reactions known that are important in white wine flavours. In the presence of oxygen, 1,1-ethanedithiol is converted to cis/trans-3,6-dimethyl-1,2,4,5-tetrathiane which has a rubbery aroma. This molecule has a ring with four sulfur atoms and two carbons, two 1,1-ethanedithiol molecules become linked at their sulfur atoms with the loss of hydrogen.[10] This can further oxidise to cis/trans-3,6-dimethyl-1,2,5-trithiolane which has a meat-like odour.[10]
1,1-Ethanedithiol reacts with hydrogen sulfide to form cis/trans-4,7-dimethyl-1,2,3,5,6-pentathiepane, a ring containing five sulfur atoms and two carbons. This has a meaty smell.[10]
Formation
1,1-Ethanedithiol can be formed in the reaction of acetaldehyde with hydrogen sulfide. 1-Hydroxyethanethiol is formed as an intermediary.[10]
See also
References
- ↑ 1.0 1.1 Smith, R. L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J. et al. (August 2005). "GRAS Flavoring Substances 22". Food Technology. Institute of Food Technologists. p. 47. http://www.ift.org/knowledge-center/focus-areas/product-development-and-ingredient-innovations/~/media/Food%20Technology/pdf/2005/08/0805feat_gras22complete.pdf. Retrieved 1 December 2012.
- ↑ 2.0 2.1 2.2 Meeting, Joint FAO/WHO Expert Committee on Food Additives.; Organization, World Health (2007). Evaluation of Certain Food Additives and Contaminants: Sixty-eighth Report of the Joint FAO/WHO Expert Committee on Food Additives Geneva from 19 to 28 June 2007. World Health Organization. pp. 108, 214–218. ISBN 9789241209472. http://whqlibdoc.who.int/publications/2007/9789241209472_eng.pdf. Retrieved 1 December 2012.
- ↑ "1,1-ethane dithiol 1% in ethanol 94.5% / ethyl acetate 4% fragrance 69382-62-3". 2018-04-15. http://www.perflavory.com/docs/doc1590721.html. Retrieved 1 December 2012.
- ↑ 4.0 4.1 Joint FAO/WHO Expert Committee on Food Additives (2012). "List of Codex Specification for Food Additives". CAC/MISC 6. p. 90. http://www.codexalimentarius.net/input/download/standards/9/CXA_006e.pdf. Retrieved 1 December 2012.
- ↑ European Food Safety Authority (2011). "Scientific Opinion on Flavouring Group Evaluation 91, Revision 1". EFSA Journal 9 (12): 2459. doi:10.2903/j.efsa.2011.2459.
- ↑ Fedrizzi, Bruno; Giuseppe Versini; Roberto Ferrarini; Fabio Finato; Giorgio Nicolini; Franco Magno (2011). "Sulfur Compounds in Still and Sparkling Wines and in Grappa: Analytical and Technological Aspects". Volatile Sulfur Compounds in Food. ACS Symposium Series. 1068. pp. 215–228. doi:10.1021/bk-2011-1068.ch010. ISBN 978-0-8412-2616-6.
- ↑ American Chemical Society (28 November 2012). "Scientists sniff out the substances behind the aroma in the 'king of fruits'". http://phys.org/news/2012-11-scientists-substances-aroma-king-fruits.html. Retrieved 1 December 2012.
- ↑ Li, Jia-Xiao; Peter Schieberle; Martin Steinhaus (2012). "Characterization of the Major Odor-Active Compounds in Thai Durian (Durio zibethinus L. 'Monthong') by Aroma Extract Dilution Analysis and Headspace Gas Chromatography–Olfactometry". Journal of Agricultural and Food Chemistry 60 (45): 11253–11262. doi:10.1021/jf303881k. ISSN 0021-8561. PMID 23088286.
- ↑ "No 1660 Ethane-1,1-dithiol HNMR". http://www.fao.org/ag/agn/jecfa-flav/img/jecfa68/1660-hnmr.gif. Retrieved 2 December 2012.
- ↑ 10.0 10.1 10.2 10.3 Moreno-Arribas, M. Victoria; Polo, Carmen (2008-11-14). Wine Chemistry and Biochemistry. Springer. pp. 604–. ISBN 9780387741161. https://books.google.com/books?id=q_AkYRM-RR8C&pg=PA604. Retrieved 2 December 2012.
External links
- "Human Metabolome Database: Showing metabocard for Ethane-1,1-dithiol (HMDB0032253)". http://www.hmdb.ca/metabolites/HMDB0032253.
- "Food safety and quality: jecfa-flav". http://www.fao.org/ag/agn/jecfa-flav/search.html?lang=en.
- Drumm, Terri D.; Arthur M. Spanier (1991). "Changes in the content of lipid autoxidation and sulfur-containing compounds in cooked beef during storage". Journal of Agricultural and Food Chemistry 39 (2): 336–343. doi:10.1021/jf00002a023. ISSN 0021-8561.