Chemistry:Triethoxysilane
| |
| Names | |
|---|---|
| Preferred IUPAC name
Triethoxysilane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H16O3Si | |
| Molar mass | 164.276 g·mol−1 |
| Appearance | colourless liquid |
| Density | 0.89 g/cm3 |
| Boiling point | 134–135 °C (273–275 °F; 407–408 K) |
| organic solvents | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
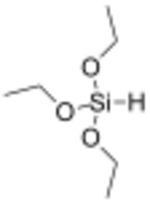
Triethoxysilane is an organosilicon compound with the formula HSi(OC2H5)3. It is a colourless liquid used in precious metal-catalysed hydrosilylation reactions. The resulting triethoxysilyl groups are often valued for attachment to silica surfaces.[1] Compared to most compounds with Si-H bonds, triethoxysilane exhibits relatively low reactivity. Like most silyl ethers, triethoxysilane is susceptible to hydrolysis. As reducing agent, triethoxysilane can for example be used in reduction of amides, reduction of carbonyl compounds in the presence of cobalt(II) chloride as catalyst, Cu-catalyzed reductive hydroxymethylation of styrenes, and Rh-catalyzed hydrodediazoniation. [2]
Applications
Compounds based on triethoxysilane might be used in fluoride varnish.[3]
References
- ↑ Robert J. P. Corriu, Christian Guérin, Karl A. Scheidt, Robert B. Lettan II, George Nikonov, Lidia Yunnikova "Triethoxysilane" e-EROS Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons doi:10.1002/047084289X.rt215.pub3
- ↑ "Triethoxysilane". https://www.organic-chemistry.org/chemicals/reductions/triethoxysilane.shtm.
- ↑ , Masato; Daisuke Takano & Masakazu Uotani et al."Fluorine-containing silane compound" patent US9926339B2, issued 2018-03-27
 |

