Chemistry:Ervogastat
From HandWiki
Short description: Experimental small-molecule drug
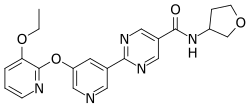 | |
| Clinical data | |
|---|---|
| Other names | PF-06865571 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H21N5O4 |
| Molar mass | 407.430 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ervogastat is an experimental small-molecule drug and selective diacylglycerol O-acyltransferase 2 inhibitor developed by Pfizer for non-alcoholic steatohepatitis.[1] Its development was previously halted by the company but resumed in 2022.[2]
References
- ↑ Futatsugi, Kentaro; Cabral, Shawn; Kung, Daniel W.; Huard, Kim; Lee, Esther; Boehm, Markus; Bauman, Jonathan; Clark, Ronald W. et al. (24 November 2022). "Discovery of Ervogastat (PF-06865571): A Potent and Selective Inhibitor of Diacylglycerol Acyltransferase 2 for the Treatment of Non-alcoholic Steatohepatitis". Journal of Medicinal Chemistry 65 (22): 15000–15013. doi:10.1021/acs.jmedchem.2c01200. PMID 36322383.
- ↑ "With the right partner, Pfizer gains fast-track tag for previously shelved NASH drug". https://www.fiercebiotech.com/biotech/right-partner-pfizer-gains-fast-track-tag-previously-shelved-nash-drug.
 |

