Chemistry:Lifitegrast
 | |
| Clinical data | |
|---|---|
| Trade names | Xiidra |
| Other names | SAR-1118 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616039 |
| Pregnancy category |
|
| Routes of administration | Eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
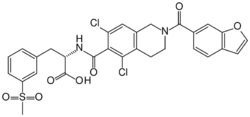
| Formula | C29H24Cl2N2O7S |
| Molar mass | 615.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lifitegrast, sold under the brand name Xiidra (/ˈzaɪdrə/[1]), is a medication for the treatment of signs and symptoms of dry eye, a syndrome called keratoconjunctivitis sicca. Lifitegrast reduces inflammation by inhibiting inflammatory cell binding.[2] It is often used in conjunction with ciclosporin (Ikervis, Restasis, or Cequa) for dry eye treatment including meibomian gland dysfunction and inflammatory dry eye.
Adverse effects
Common side effects in clinical trials were eye irritation, discomfort, blurred vision, and dysgeusia (a distortion of the sense of taste).[3]
Pharmacology
Lifitegrast is supplied as an eye drop.
Mechanism of action
Lifitegrast inhibits an integrin, lymphocyte function-associated antigen 1 (LFA-1), from binding to intercellular adhesion molecule 1 (ICAM-1). This mechanism down-regulates inflammation mediated by T lymphocytes.[2][4]
History
Lifitegrast was initially designed by Sunesis and developed by SARcode Bioscience[5] which was acquired by Shire in 2013,[6] which submitted a new drug application to the US Food and Drug Administration (FDA) in March 2015. The FDA granted Shire a priority review a month later, and requested additional clinical data, which were supplied in January 2016; approval was granted on 11 July 2016.[7][8] Lifitegrast was approved by Health Canada in January 2018, and available in Canadian pharmacies as of March 2018.
Shire was acquired by Takeda Pharmaceutical Company in late 2018.[9] In May 2019 Novartis reached an agreement to purchase the assets associated with Lifitegrast. Novartis will pay Takeda an upfront payment of $3.4 billion, while the latter drugmaker is eligible for milestone payments of as much as $1.9 billion. Novartis noted that the drug amassed approximately $400 million in revenue in 2018.[10] In 2023, Novartis sold the assets to Bausch + Lomb for $1.75 billion and eligible for an additional $750 million in payments linked to future sales for Xiidra as well as two pipeline assets.[11][12]
References
- ↑ "Patient information: Xiidra® (ZYE-druh) (lifitegrast ophthalmic solution) 5% for topical ophthalmic use". Novartis. June 2020. https://www.novartis.us/sites/www.novartis.us/files/xiidra_ppi.pdf.
- ↑ 2.0 2.1 "Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study". Ophthalmology 122 (12): 2423–2431. December 2015. doi:10.1016/j.ophtha.2015.08.001. PMID 26365210.
- ↑ Drugs.com: Patient information for xiidra.
- ↑ "The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs". Investigative Ophthalmology & Visual Science 52 (6): 3174–3180. May 2011. doi:10.1167/iovs.09-5078. PMID 21330663.
- ↑ "Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease". Clinical Ophthalmology 10: 1083–1094. 2016. doi:10.2147/OPTH.S110557. PMID 27354762.
- ↑ "Shire To Acquire Sarcode Bioscience, Expands Presence In Ophthalmology". Shire. 25 March 2013. https://www.shire.com/en/newsroom/2013/march/shire-to-acquire-sarcode.
- ↑ "FDA Approves Shire's Xiidra". 11 July 2016. https://www.shire.com/newsroom/2016/july/9pks5v.
- ↑ "Xiidra (lifitegrast) FDA Approval History". Drugs.com. https://www.drugs.com/history/xiidra.html.
- ↑ "Takeda Completes Acquisition of Shire, Becoming a Global, Values-based, R&D-Driven Biopharmaceutical Leader". Takeda. 8 January 2019. https://www.takeda.com/newsroom/newsreleases/2019/takeda-completes-acquisition-of-shire-becoming-a-global-values-based-rd-driven-biopharmaceutical-leader/.
- ↑ "Novartis to acquire Xiidra, expanding front-of-eye portfolio and strengthening leadership in eye care". Novartis (Press release). 9 May 2019.
- ↑ "Novartis Sells Eye Drugs to Bausch + Lomb for Up to $2.5 Billion" (in en). Bloomberg News. 30 June 2023. https://www.bloomberg.com/news/articles/2023-06-30/novartis-sells-eye-drugs-to-bausch-lomb-for-up-to-2-5-billion.
- ↑ "Bausch + Lomb closes $2.5B XIIDRA acquisition". NJBIZ. 4 October 2023. https://njbiz.com/bausch-lomb-closes-2-5b-xiidra-acquisition/.
 |

