Chemistry:Sterol
| |
| Names | |
|---|---|
| IUPAC name
2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17-hexadecahydro-1H-cyclopenta[a]phenanthren-3-ol
| |
| Other names
Hexadecahydro-3H-cyclopenta[a]phenanthrene-3-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C17H28O | |
| Molar mass | 248.410 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
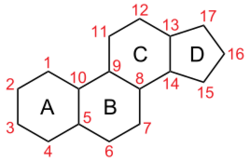
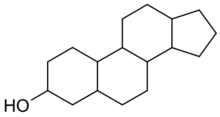
Sterol is an organic compound[1] with formula C17H28O, whose molecule is derived from that of gonane by replacement of a hydrogen atom on C3 position by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions).[2][3] The most familiar type of animal sterol is cholesterol, which is vital to cell membrane structure, and functions as a precursor to fat-soluble vitamins and steroid hormones.
While technically alcohols, sterols are classified by biochemists as lipids (fats in the broader sense of the term).
Types
Phytosterols
Phytosterols are sterols naturally found in plants. Notable examples of phytosterols include campesterol, sitosterol, and stigmasterol.
Zoosterols
Zoosterols are sterols found in animals. The most significant zoosterol is cholesterol.
Mycosterols
Sterols found in fungi are called mycosterols. A common example is ergosterol, a mycosterol present in the cell membrane of fungi, where it serves a role similar to cholesterol in animal cells.
Role in biochemistry
Sterols and related compounds play essential roles in the physiology of eukaryotic organisms, and are essential for normal physiology of plants, animals, and fungi.[4] For example, cholesterol forms part of the cellular membrane in animals, where it affects the cell membrane's fluidity and serves as secondary messenger in developmental signaling. In humans and other animals, corticosteroids such as cortisol act as signaling compounds in cellular communication and general metabolism. Sterols are common components of human skin oils.[5]
Phytosterols as a nutritional supplement
Phytosterols, more commonly known as plant sterols, have been shown in clinical trials to block cholesterol absorption sites in the human intestine, thus helping to reduce cholesterol absorption in humans.[6] They are currently approved by the U.S. Food and Drug Administration for use as a food supplement; however, there is some concern that they may block absorption not only of cholesterol, but of other important nutrients as well. At present, the American Heart Association has recommended that supplemental plant sterols be taken only by those diagnosed with elevated cholesterol, and has particularly recommended that they not be taken by pregnant women or nursing mothers.[7] Preliminary research has shown that phytosterols may have anticancer effects.[8]
Chemical classification and structure
Sterols are a subgroup of steroids with a hydroxyl group at the 3-position of the A-ring.[9] They are amphipathic lipids synthesized from acetyl-coenzyme A via the HMG-CoA reductase pathway. The overall molecule is quite flat. The hydroxyl group on the A ring is polar. The rest of the aliphatic chain is non-polar.
See also
- Cholesterol
- Ergosterol
- Hopanoids
- Hydroxysteroid
- Phytosterol
- Steroids
- Zoosterol
References
- ↑ "sterol (CHEBI:15889)". https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:15889#:~:text=Sterol%20is%20an%20organic%20compound,therefore%20an%20alcohol%20of%20gonane..
- ↑ "Sterol Synthesis in Diverse Bacteria". Frontiers in Microbiology 7: 990. 24 June 2016. doi:10.3389/fmicb.2016.00990. PMID 27446030.
- ↑ "Evolution of bacterial steroid biosynthesis and its impact on eukaryogenesis". Proceedings of the National Academy of Sciences of the United States of America 118 (25): e2101276118. June 2021. doi:10.1073/pnas.2101276118. PMID 34131078. Bibcode: 2021PNAS..11801276H.
- ↑ Luo, Jie; Yang, Hongyuan; Song, Bao-Liang (April 2020). "Mechanisms and regulation of cholesterol homeostasis" (in en). Nature Reviews Molecular Cell Biology 21 (4): 225–245. doi:10.1038/s41580-019-0190-7. ISSN 1471-0080. PMID 31848472. https://www.nature.com/articles/s41580-019-0190-7.
- ↑ "Human stratum corneum lipids: characterization and regional variations". Journal of Lipid Research 24 (2): 120–30. February 1983. doi:10.1016/S0022-2275(20)38005-6. PMID 6833889.
- ↑ "Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ". The American Journal of Clinical Nutrition 77 (6): 1385–9. June 2003. doi:10.1093/ajcn/77.6.1385. PMID 12791614.
- ↑ "Do we need to be buying plant sterols?". Food Magazine 67: 14. October–December 2004. http://www.foodcomm.org.uk/17.pdf. Retrieved 8 August 2008.
- ↑ "Phytosterols as anticancer compounds". Molecular Nutrition & Food Research 51 (2): 161–70. February 2007. doi:10.1002/mnfr.200600164. PMID 17266177.
- ↑ "A comprehensive classification system for lipids". Journal of Lipid Research 46 (5): 839–61. May 2005. doi:10.1194/jlr.E400004-JLR200. PMID 15722563.
Further reading
- "10. Membrane Structure". Molecular biology of the cell. IV. Internal Organization of the Cell. New York: Garland Science. 2002. p. 1874. ISBN 978-0-8153-4072-0. https://www.ncbi.nlm.nih.gov/books/bv.fcgi?highlight=cholesterol,bilayer&rid=mboc4.section.1864#1874. "The Fluidity of a Lipid Bilayer Depends on Its Composition"
External links
- Sterols Cyberlipid.org
 |