Chemistry:Hydroxy group

In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula –OH and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion HO−
, called hydroxide, and the neutral radical HO · , known as the hydroxyl radical, consist of an unbonded hydroxy group.
According to IUPAC definitions, the term hydroxyl refers to the hydroxyl radical ( · OH) only, while the functional group –OH is called a hydroxy group.[1]
Properties
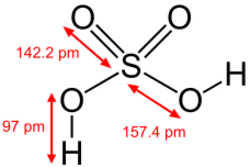
Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack this functional group. Organic compounds, which are often poorly soluble in water, become water-soluble when they contain two or more hydroxy groups, as illustrated by sugars and amino acid.[citation needed]
Occurrence
The hydroxy group is pervasive in chemistry and biochemistry. Many inorganic compounds contain hydroxyl groups, including sulfuric acid, the chemical compound produced on the largest scale industrially.[2]
Hydroxy groups participate in the dehydration reactions that link simple biological molecules into long chains. The joining of a fatty acid to glycerol to form a triacylglycerol removes the −OH from the carboxy end of the fatty acid. The joining of two aldehyde sugars to form a disaccharide removes the −OH from the carboxy group at the aldehyde end of one sugar. The creation of a peptide bond to link two amino acids to make a protein removes the −OH from the carboxy group of one amino acid.[3]
Hydroxyl radical
Hydroxyl radicals are highly reactive and undergo chemical reactions that make them short-lived. When biological systems are exposed to hydroxyl radicals, they can cause damage to cells, including those in humans, where they can react with DNA, lipids, and proteins.[4]
Planetary observations
Airglow of the Earth
The Earth's night sky is illuminated by diffuse light, called airglow, that is produced by radiative transitions of atoms and molecules.[5] Among the most intense such features observed in the Earth's night sky is a group of infrared transitions at wavelengths between 700 nanometers and 900 nanometers. In 1950, Aden Meinel showed that these were transitions of the hydroxyl molecule, OH.[6]
Surface of the Moon
In 2009, India's Chandrayaan-1 satellite and the National Aeronautics and Space Administration (NASA) Cassini spacecraft and Deep Impact probe each detected evidence of water by evidence of hydroxyl fragments on the Moon. As reported by Richard Kerr, "A spectrometer [the Moon Mineralogy Mapper, also known as "M3"] detected an infrared absorption at a wavelength of 3.0 micrometers that only water or hydroxyl—a hydrogen and an oxygen bound together—could have created."[7] NASA also reported in 2009 that the LCROSS probe revealed an ultraviolet emission spectrum consistent with hydroxyl presence.[8]
On 26 October 2020, NASA reported definitive evidence of water on the sunlit surface of the Moon, in the vicinity of the crater Clavius (crater), obtained by the Stratospheric Observatory for Infrared Astronomy (SOFIA).[9] The SOFIA Faint Object infrared Camera for the SOFIA Telescope (FORCAST) detected emission bands at a wavelength of 6.1 micrometers that are present in water but not in hydroxyl. The abundance of water on the Moon's surface was inferred to be equivalent to the contents of a 12-ounce bottle of water per cubic meter of lunar soil.[10]
The Chang'e 5 probe, which landed on the Moon on 1 December 2020, carried a mineralogical spectrometer that could measure infrared reflectance spectra of lunar rock and regolith. The reflectance spectrum of a rock sample at a wavelength of 2.85 micrometers indicated localized water/hydroxyl concentrations as high as 180 parts per million.[11]
Atmosphere of Venus
The Venus Express orbiter collected Venus science data from April 2006 until December 2014. In 2008, Piccioni, et al. reported measurements of night-side airglow emission in the atmosphere of Venus made with the Visible and Infrared Thermal Imaging Spectrometer (VIRTIS) on Venus Express. They attributed emission bands in wavelength ranges of 1.40 - 1.49 micrometers and 2.6 - 3.14 micrometers to vibrational transitions of OH.[12] This was the first evidence for OH in the atmosphere of any planet other than Earth's.[12]
Atmosphere of Mars
In 2013, OH near-infrared spectra were observed in the night glow in the polar winter atmosphere of Mars by use of the Compact Reconnaissance Imaging Spectrometer for Mars (CRISM).[13]
Exoplanets
In 2021, evidence for OH in the dayside atmosphere of the exoplanet WASP-33b was found in its emission spectrum at wavelengths between 1 and 2 micrometers.[14] Evidence for OH in the atmosphere of exoplanet WASP-76b was subsequently found.[15] Both WASP-33b and WASP-76b are ultra-hot Jupiters and it is likely that any water in their atmospheres is present as dissociated ions.
See also
References
- ↑ Alcohols. IUPAC. February 24, 2014. doi:10.1351/goldbook.A00204. http://goldbook.iupac.org/A00204.html. Retrieved 23 March 2015.
- ↑ "Research Report 2012 – 2013". Ludwig Maximilians Universität München Fakultät für Chemie und Pharmazie 12. https://www.cup.uni-muenchen.de/site/assets/files/1039/fob_bd_12.pdf.
- ↑ "Peptide Bond - an overview". https://www.sciencedirect.com/topics/engineering/peptide-bond.
- ↑ Kanno, Taro; Nakamura, Keisuke; Ikai, Hiroyo; Kikuchi, Katsushi; Sasaki, Keiichi; Niwano, Yoshimi (July 2012). "Literature review of the role of hydroxyl radicals in chemically-induced mutagenicity and carcinogenicity for the risk assessment of a disinfection system utilizing photolysis of hydrogen peroxide". Journal of Clinical Biochemistry and Nutrition 51 (1): 9–14. doi:10.3164/jcbn.11-105. ISSN 0912-0009. PMID 22798706.
- ↑ "Night airglow phenomenology.". Space Science Reviews 11 (2): 341–79. October 1970. doi:10.1007/BF00241526. Bibcode: 1970SSRv...11..341S. http://adsabs.harvard.edu/full/1970SSRv...11..341S.
- ↑ "OH Emission Bands in the Spectrum of the Night Sky. I". Astrophysical Journal 111: 555–564. 1950. doi:10.1086/145296. Bibcode: 1950ApJ...111..555M. http://articles.adsabs.harvard.edu/pdf/1950ApJ...111..555M.
- ↑ "A Whiff of Water Found on the Moon". Science. 24 September 2009. https://www.science.org/content/article/whiff-water-found-moon.
- ↑ "LCROSS Impact Data Indicates Water on Moon". NASA. 13 November 2009. http://www.nasa.gov/mission_pages/LCROSS/main/prelim_water_results.html.
- ↑ "Molecular water detected on the sunlit Moon by SOFIA". Nature Astronomy 5 (2): 121–127. 2020. doi:10.1038/s41550-020-01222-x. Bibcode: 2021NatAs...5..121H.
- ↑ "NASA's SOFIA Discovers Water on Sunlit Surface of Moon". NASA. 26 October 2020. https://www.nasa.gov/press-release/nasa-s-sofia-discovers-water-on-sunlit-surface-of-moon/.
- ↑ "In situ detection of water on the Moon by the Chang'E-5 lander". Science Advances 8 (1): eabl9174. 2022. doi:10.1126/sciadv.abl9174. PMID 34995111. Bibcode: 2022SciA....8.9174L.
- ↑ 12.0 12.1 Piccioni, G.; Drossart, P.; Zasova, L.; Migliorini, A.; Gérard, J.-C.; Mills, F. P.; Shakun, A.; García Muñoz, A. et al. (2008-04-01). "First detection of hydroxyl in the atmosphere of Venus". Astronomy & Astrophysics (EDP Sciences) 483 (3): L29–L33. doi:10.1051/0004-6361:200809761. ISSN 0004-6361.
- ↑ "First detection of Mars atmospheric hydroxyl: CRISM Near-IR measurement versus LMD GCM simulation of OH Meinel band emission in the Mars polar winter atmosphere". Icarus 226 (1): 272–281. 2013. doi:10.1016/j.icarus.2013.05.035. Bibcode: 2013Icar..226..272T.
- ↑ Stevanus K. Nugroho; Hajime Kawahara; Neale P. Gibson; Ernst J. W. de Mooij; Teruyuki Hirano; Takayuki Kotani; Yui Kawashima; Kento Masuda et al. (2021). "First Detection of Hydroxyl Radical Emission from an Exoplanet Atmosphere: High-dispersion Characterization of {WASP}-33b Using Subaru/{IRD}". Astrophysical Journal Letters 910 (1): L9. doi:10.3847/2041-8213/abec71. https://pureadmin.qub.ac.uk/ws/files/237491214/2103.03094v1.pdf.
- ↑ R. Landman; A. Sánchez-López; P. Mollière; A. Y. Kesseli; A. J. Louca; I. A. G. Snellen (2021). "Detection of OH in the ultra-hot Jupiter WASP-76b". Astronomy and Astrophysics 656 (1): A119. doi:10.1051/0004-6361/202141696. Bibcode: 2021A&A...656A.119L.
Further
- "Chapter 4&5". Campbell Biology. Unit 1 (9th ed.). San Francisco: Pearson Benjamin Cummings.. 2011. ISBN 978-0-321-55823-7. https://books.google.com/books?id=39vMSgAACAAJ.
External links
 |
