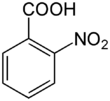
Chemistry:2-Nitrobenzoic acid
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Nitrobenzoic acid | |||
| Other names
o-Nitrobenzoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H5NO4 | |||
| Molar mass | 167.12 g/mol | ||
| Density | 1.468 | ||
| Melting point | 147.5 °C (297.5 °F; 420.6 K) | ||
| Acidity (pKa) | 2.16 | ||
| -76.11·10−6 cm3/mol | |||
| Related compounds | |||
Related compounds
|
Benzoic acid Nitrobenzene Anthranilic acid 3,5-Dinitrobenzoic acid 3-Nitrobenzoic acid 4-Nitrobenzoic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2-Nitrobenzoic acid or o-nitrobenzoic acid is an organic compound with the formula C6H4(NO2)CO2H. It is prepared by oxidation of 2-nitrotoluene with nitric acid.[1] In consists of a carboxylic acid group and a nitro group in the ortho configuration. Reduction of the nitro group into an amine produces anthranilic acid.
References
- ↑ Takao Maki, Kazuo Takeda "Benzoic Acid and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_555.
 |