Biology:Barnase
| Barnase | |
|---|---|
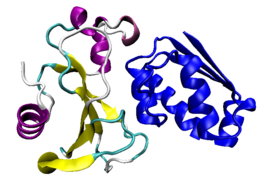 The tightly bound complex between barnase and its inhibitor barstar. Barnase is colored by secondary structure and barstar is colored in blue.[1] | |
| Identifiers | |
| Symbol | Barnase |
| PDB | 1BRS More structures |
| UniProt | P00648 |
| Other data | |
| EC number | 3.1.27.- |
Barnase (a portmanteau of "BActerial" "RiboNucleASE") is a bacterial protein that consists of 110 amino acids and has ribonuclease activity. It is synthesized and secreted by the bacterium Bacillus amyloliquefaciens, but is lethal to the cell when expressed without its inhibitor barstar. The inhibitor binds to and occludes the ribonuclease active site, preventing barnase from damaging the cell's RNA after it has been synthesized but before it has been secreted. The barnase/barstar complex is noted for its extraordinarily tight protein-protein binding, with an on-rate of 108s−1M−1.
Protein folding studies
Barnase has no disulfide bonds, nor does it require divalent cations or non-peptide components to fold. This simplicity, in combination with its reversible folding transition, means that barnase has been extensively studied in order to understand how proteins fold.[2][3][4] The folding of barnase has been extensively studied in the laboratory of Alan Fersht, who used it as the test case in developing a method of characterizing protein folding transition states known as phi value analysis.
Active site and catalytic mechanism
Barnase catalyzes hydrolysis at diribonucleotide GpN sites. Cleavage occurs in two steps using a general acid-base mechanism: a cyclic intermediate is formed during the first transesterification step, which is then hydrolysed to release the cleaved RNA. The two most important residues involved in catalysis are Glu73 and His102, which are both essential for enzymatic activity. Glu73 is the general base whilst His102 is the general acid. Although it is not directly involved in acid-base catalysis, Lys27 is also critical for activity; it has been implicated in transition-state substrate binding.[5]
See also
References
- ↑ PDB: 1BRS; "Protein-protein recognition: crystal structural analysis of a barnase-barstar complex at 2.0-A resolution". Biochemistry 33 (30): 8878–8889. August 1994. doi:10.1021/bi00196a004. PMID 8043575.
- ↑ "The folding of an enzyme. II. Substructure of barnase and the contribution of different interactions to protein stability". Journal of Molecular Biology 224 (3): 783–804. April 1992. doi:10.1016/0022-2836(92)90562-X. PMID 1569557.
- ↑ "The folding of an enzyme. III. Structure of the transition state for unfolding of barnase analysed by a protein engineering procedure". Journal of Molecular Biology 224 (3): 805–818. April 1992. doi:10.1016/0022-2836(92)90563-Y. PMID 1569558.
- ↑ "The folding of an enzyme. IV. Structure of an intermediate in the refolding of barnase analysed by a protein engineering procedure". Journal of Molecular Biology 224 (3): 819–835. April 1992. doi:10.1016/0022-2836(92)90564-Z. PMID 1569559.
- ↑ "Kinetic characterization of the recombinant ribonuclease from Bacillus amyloliquefaciens (barnase) and investigation of key residues in catalysis by site-directed mutagenesis". Biochemistry 28 (9): 3843–3850. May 1989. doi:10.1021/bi00435a033. PMID 2665810.
Further reading
- "Toward solving the folding pathway of barnase: the complete backbone 13C, 15N, and 1H NMR assignments of its pH-denatured state". Proceedings of the National Academy of Sciences of the United States of America 91 (20): 9412–9416. September 1994. doi:10.1073/pnas.91.20.9412. PMID 7937780. Bibcode: 1994PNAS...91.9412A.
- "A comparison of the pH, urea, and temperature-denatured states of barnase by heteronuclear NMR: implications for the initiation of protein folding". Journal of Molecular Biology 254 (2): 305–321. November 1995. doi:10.1006/jmbi.1995.0618. PMID 7490750.
- "pKA values of carboxyl groups in the native and denatured states of barnase: the pKA values of the denatured state are on average 0.4 units lower than those of model compounds". Biochemistry 34 (29): 9424–9433. July 1995. doi:10.1021/bi00029a018. PMID 7626612.
External links
 |

