Biology:Nylon-eating bacteria
| Nylon-eating bacteria | |
|---|---|
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Actinomycetota |
| Class: | Actinomycetia |
| Order: | Micrococcales |
| Family: | Micrococcaceae |
| Genus: | Paenarthrobacter |
| Species: | |
| Variety: | P. u. var. KI72
|
| Trinomial name | |
| Paenarthrobacter ureafaciens var. KI72 GTDB r95 & NCBI, 2020 (Busse HJ, 2016)
| |
| Synonyms | |
(Due to an OCR error, the strain name has occasionally been reported as "K172".) | |
Paenarthrobacter ureafaciens KI72, popularly known as nylon-eating bacteria, is a strain of Paenarthrobacter ureafaciens that can digest certain by-products of nylon 6 manufacture.[2] It uses a set of enzymes to digest nylon, popularly known as nylonase.[3]
Discovery and nomenclature
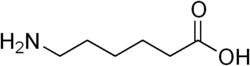
In 1975, a team of Japanese scientists discovered a strain of bacterium, living in ponds containing waste water from a nylon factory, that could digest certain byproducts of nylon 6 manufacture, such as the linear dimer of 6-aminohexanoate. These substances are not known to have existed before the invention of nylon in 1935. It was initially named as Achromobacter guttatus.[4]
Studies in 1977 revealed that the three enzymes that the bacteria were using to digest the byproducts were significantly different from any other enzymes produced by any other bacteria, and not effective on any material other than the manmade nylon byproducts.[5]
The bacterium was reassigned to Flavobacterium in 1980.[6] Its genome was resolved in 2017, again reassigning it to Arthrobacter.[1] The Genome Taxonomy Database considers it a strain of Paenarthrobacter ureafaciens following a 2016 reclassification.[7] As of January 2021, the NCBI taxonomy browser has been updated to match GTDB.
Descendant strains
A few newer strains have been created by growing the original KI72 in different conditions, forcing it to adapt. These include KI722, KI723, KI723T1, KI725, KI725R, and many more.[8]
The enzymes
The bacterium contains the following three enzymes:
- 6-aminohexanoate-cyclic-dimer hydrolase (EI, NylA, P13398)
- 6-aminohexanoate-dimer hydrolase (EII, NylB, P07061)
- 6-aminohexanoate-oligomer endohydrolase (EIII, NylC, Q57326)
All three enzymes are encoded on a plasmid called pOAD2.[9] The plasmid can be transferred to E. coli, as shown in a 1983 publication.[10]
EI
The enzyme EI is related to amidases. Its structure was resolved in 2010.[11]
EII
EII has evolved by gene duplication followed by base substitution of another protein EII'. Both enzymes have 345 identical aminoacids out of 392 aminoacids (88% homology). The enzymes are similar to beta-lactamase.[12]
The EII' (NylB', P07062) protein is about 100x times less efficient compared to EII. A 2007 research by the Seiji Negoro team shows that just two amino-acid alterations to EII', i.e. G181D and H266N, raises its activity to 85% of EII.[9]
EIII
The structure of EIII was resolved in 2018. Instead of being a completely novel enzyme, it appears to be a member of the N-terminal nucleophile (N-tn) hydrolase family.[13] Specifically, computational approaches classify it as a MEROPS S58 (now renamed P1) hydrolase. The protein is expressed as a precursor, which then cleaves itself into two chains.[14][15] Outside of this plasmid, > 95% similar proteins are found in Agromyces and Kocuria.[13]
EIII was originally thought to be completely novel. Susumu Ohno proposed that it had come about from the combination of a gene-duplication event with a frameshift mutation. An insertion of thymidine would turn an arginine-rich 427aa protein into this 392aa enzyme.[16]
Role in evolution teaching
There is scientific consensus that the capacity to synthesize nylonase most probably developed as a single-step mutation that survived because it improved the fitness of the bacteria possessing the mutation. More importantly, one of the enzymes involved was produced by a frame-shift mutation that completely scrambled existing genetic code data.[17] Despite this, the new gene still had a novel, albeit weak, catalytic capacity. This is seen as a good example of how mutations easily can provide the raw material for evolution by natural selection.[18][19][20][21]
A 1995 paper showed that scientists have also been able to induce another species of bacterium, Pseudomonas aeruginosa, to evolve the capability to break down the same nylon byproducts in a laboratory by forcing them to live in an environment with no other source of nutrients.[22]
See also
- Biodegradable plastic
- E. coli long-term evolution experiment
- Radiotrophic fungus
- London Underground mosquito
- Lonicera fly
- Mealworms are capable of digesting polystyrene
References
- ↑ 1.0 1.1 Takehara, I; Kato, DI; Takeo, M; Negoro, S (27 April 2017). "Draft Genome Sequence of the Nylon Oligomer-Degrading Bacterium Arthrobacter sp. Strain KI72.". Genome Announcements 5 (17). doi:10.1128/genomeA.00217-17. PMID 28450506.
- ↑ Takehara, I; Fujii, T; Tanimoto, Y (Jan 2018). "Metabolic pathway of 6-aminohexanoate in the nylon oligomer-degrading bacterium Arthrobacter sp. KI72: identification of the enzymes responsible for the conversion of 6-aminohexanoate to adipate". Applied Microbiology and Biotechnology 102 (2): 801–814. doi:10.1007/s00253-017-8657-y. PMID 29188330.
- ↑ Michael Le Page (March 2009). "Five classic examples of gene evolution". https://www.newscientist.com/article/dn16834-five-classic-examples-of-gene-evolution/.
- ↑ Kinoshita, S.; Kageyama, S.; Iba, K.; Yamada, Y.; Okada, H. (1975). "Utilization of a cyclic dimer and linear oligomers of e-aminocaproic acid by Achromobacter guttatus KI 72". Agricultural and Biological Chemistry 39 (6): 1219–23. doi:10.1271/bbb1961.39.1219. ISSN 0002-1369.
- ↑ S, Kinoshita; S, Negoro; M, Muramatsu; Vs, Bisaria; S, Sawada; H, Okada (1977-11-01). "6-Aminohexanoic Acid Cyclic Dimer Hydrolase. A New Cyclic Amide Hydrolase Produced by Achromobacter Guttatus KI74" (in en). European Journal of Biochemistry 80 (2): 489–95. doi:10.1111/j.1432-1033.1977.tb11904.x. PMID 923591.
- ↑ Negoro, S; Shinagawa, H; Nakata, A; Kinoshita, S; Hatozaki, T; Okada, H (July 1980). "Plasmid control of 6-aminohexanoic acid cyclic dimer degradation enzymes of Flavobacterium sp. KI72.". Journal of Bacteriology 143 (1): 238–45. doi:10.1128/JB.143.1.238-245.1980. PMID 7400094.
- ↑ "GTDB - GCF_002049485.1". 2020. https://gtdb.ecogenomic.org/genomes?gid=GCF_002049485.1.
- ↑ Negoro, S; Kakudo, S; Urabe, I; Okada, H (1992). "A new nylon oligomer degradation gene (nylC) on plasmid pOAD2 from a Flavobacterium sp.". Journal of Bacteriology 174 (24): 7948–7953. doi:10.1128/jb.174.24.7948-7953.1992. PMID 1459943.
- ↑ 9.0 9.1 "Nylon-oligomer degrading enzyme/substrate complex: catalytic mechanism of 6-aminohexanoate-dimer hydrolase". J. Mol. Biol. 370 (1): 142–56. June 2007. doi:10.1016/j.jmb.2007.04.043. PMID 17512009.
- ↑ "Plasmid-determined enzymatic degradation of nylon oligomers". J. Bacteriol. 155 (1): 22–31. July 1983. doi:10.1128/JB.155.1.22-31.1983. PMID 6305910.
- ↑ Yasuhira, K; Shibata, N; Mongami, G; Uedo, Y; Atsumi, Y; Kawashima, Y; Hibino, A; Tanaka, Y et al. (8 January 2010). "X-ray crystallographic analysis of the 6-aminohexanoate cyclic dimer hydrolase: catalytic mechanism and evolution of an enzyme responsible for nylon-6 byproduct degradation.". The Journal of Biological Chemistry 285 (2): 1239–48. doi:10.1074/jbc.M109.041285. PMID 19889645.
- ↑ Okada, H.; Negoro, S.; Kimura, H.; Nakamura, S. (10–16 November 1983). "Evolutionary adaptation of plasmid-encoded enzymes for degrading nylon oligomers". Nature 306 (5939): 203–206. doi:10.1038/306203a0. ISSN 0028-0836. PMID 6646204. Bibcode: 1983Natur.306..203O.
- ↑ 13.0 13.1 Negoro, S; Shibata, N; Lee, YH; Takehara, I; Kinugasa, R; Nagai, K; Tanaka, Y; Kato, DI et al. (27 June 2018). "Structural basis of the correct subunit assembly, aggregation, and intracellular degradation of nylon hydrolase.". Scientific Reports 8 (1): 9725. doi:10.1038/s41598-018-27860-w. PMID 29950566. Bibcode: 2018NatSR...8.9725N.
- ↑ "Q57326". https://www.ebi.ac.uk/interpro/protein/UniProt/Q57326/.
- ↑ "MEROPS - the Peptidase Database". https://www.ebi.ac.uk/merops/cgi-bin/pepsum?id=P01.102.
- ↑ Ohno S (April 1984). "Birth of a unique enzyme from an alternative reading frame of the preexisted, internally repetitious coding sequence". Proc Natl Acad Sci USA 81 (8): 2421–5. doi:10.1073/pnas.81.8.2421. PMID 6585807. Bibcode: 1984PNAS...81.2421O.
- ↑ Ohno, S (April 1984). "Birth of a unique enzyme from an alternative reading frame of the preexisted, internally repetitious coding sequence." (in en). Proceedings of the National Academy of Sciences 81 (8): 2421–2425. doi:10.1073/pnas.81.8.2421. ISSN 0027-8424. PMID 6585807. PMC 345072. https://pnas.org/doi/full/10.1073/pnas.81.8.2421.
- ↑ Thwaites WM (Summer 1985). "New Proteins Without God's Help". Creation Evolution Journal 5 (2): 1–3. http://ncse.com/cej/5/2/new-proteins-without-gods-help.
- ↑ "Evolution and Information: The Nylon Bug". New Mexicans for Science Education. http://www.nmsr.org/nylon.htm.
- ↑ Than, Ker (2005-09-23). "Why scientists dismiss 'intelligent design'" (in en). https://www.nbcnews.com/id/wbna9452500.
- ↑ Miller, Kenneth R. Only a Theory: Evolution and the Battle for America's Soul (2008) pp. 80-82
- ↑ "Emergence of nylon oligomer degradation enzymes in Pseudomonas aeruginosa PAO through experimental evolution". Appl. Environ. Microbiol. 61 (5): 2020–2. May 1995. doi:10.1128/AEM.61.5.2020-2022.1995. PMID 7646041. Bibcode: 1995ApEnM..61.2020P.
- "No stop codons in the antisense strands of the genes for nylon oligomer degradation". Proc Natl Acad Sci USA 89 (9): 3780–4. May 1992. doi:10.1073/pnas.89.9.3780. PMID 1570296. Bibcode: 1992PNAS...89.3780Y.
External links
- NBRC 14590, information on the KI72 culture maintained at National Institute of Technology and Evaluation
- NBRC 114184, a derived culture used in the 2017 sequencing
- GO:0019876: Nylon catabolic process
Wikidata ☰ Q4353307 entry
 |

