Chemistry:2-Methyltetrahydroquinoline
From HandWiki
Revision as of 22:04, 30 June 2021 by imported>Jworkorg (fixing)
| |
| Names | |
|---|---|
| Other names
Tetrahydroquinaldine, 1,2,3,4-Tetrahydroquinaldine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL |
|
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H13N | |
| Molar mass | 147.221 g·mol−1 |
| Appearance | colorless oil |
| Boiling point | 125 °C (257 °F; 398 K) (17 Torr) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319 | |
| P264, P280, P302+352, P305+351+338, P321, P332+313, P337+313, P362 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
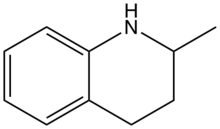
2-Methyltetrahydroquinoline is one of the methyl-substituted derivatives of tetrahydroquinoline. A colorless oil, it is a chiral compound owing to the presence of the methyl substituent. It is produced by the hydrogenation of quinaldine.[1] It is of interest in medicinal chemistry.[2]
References
- ↑ Chakraborty, Sumit; Brennessel, William W.; Jones, William D. (2014). "A Molecular Iron Catalyst for the Acceptorless Dehydrogenation and Hydrogenation of N-Heterocycles". Journal of the American Chemical Society 136 (24): 8564–8567. doi:10.1021/ja504523b. PMID 24877556.
- ↑ Sridharan, Vellaisamy; Suryavanshi, Padmakar A.; Menéndez, J. Carlos (2011). "Advances in the Chemistry of Tetrahydroquinolines". Chemical Reviews 111 (11): 7157–7259. doi:10.1021/cr100307m. PMID 21830756.

