Chemistry:Metallaborane
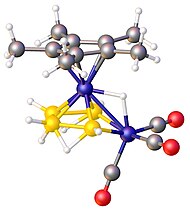
5(CH
3)
5)FeHCo(CO)
3B
4H
7.[1] Color code: yellow = B, blue = Fe & Co, red = O, gray = C.
In chemistry, a metallaborane is a compound that contains one or more metal atoms and one or more boron hydride. These compounds are related conceptually and often synthetically to the boron-hydride clusters by replacement of BHn units with metal-containing fragments. Often these metal fragments are derived from metal carbonyls or cyclopentadienyl complexes. Their structures can often be rationalized by polyhedral skeletal electron pair theory. The inventory of these compounds is large, and their structures can be quite complex. [2][3]
Examples
Two simple examples are B
4H
8Fe(CO)
3 and B
4H
8Co(C
5H
5). The MB4 cores (M = Fe or Co) of these two compounds adopt structures expected for nido 5-vertex clusters. The iron compound is produced by reaction of diiron nonacarbonyl with pentaborane. B
4H
8Fe(CO)
3 and cyclobutadieneiron tricarbonyl have similar structures.
Metallacarboranes
Even greater in scope than metalloboranes are metallacarboranes. These cages have carbon vertices, often CH, in addition to BH and M vertices.[2] A well-developed class of metallacarboranes are prepared from dicarbollides, anions of the formula [C2B9H11]2-. These anions function as ligands for a variety of metals, often forming sandwich complexes.[5]
Some metalloboranes are derived by the metalation of neutral carboranes. Illustrative are the six-and seven-vertex cages prepared from closo-C
2B
3H
5. Reaction of this carborane with iron carbonyl sources gives closo Fe- and Fe2-containing products, according to these idealized equations:[6]
- C
2B
3H
5 + Fe
2(CO)
9 → C
2B
3H
5Fe(CO)
3 + Fe(CO)
5 + CO - C
2B
3H
5Fe(CO)
3 + Fe
2(CO)
9 → C
2B
3H
5(Fe(CO)
3)
2 + Fe(CO)
5 + CO
A further example of insertion into a closo carborane is the synthesis of the yellow-orange solid closo-1,2,3-(CO)
3FeC
2B
4H
6:
- closo–C
2B
4H
8 + Fe
2(CO)
9 → closo–(CO)
3FeC
2B
4H
6 + Fe(CO)
5 + CO
A closely related reaction involves the capping of an anionic nido carborane C
2B
4H−
7
- closo–C
2B
4H
8 + NaH → Na(nido–B
4H
7) + H
2 - Na(nido–B
4H
7 + CoCl
2 + NaC
5H
5 → closo–(C
5H
5(CoB
4H
6 + 2 NaCl + ...
The last reaction is worked up with acid and air.
References
- ↑ Peldo, Melanie A.; Beatty, Alicia M.; Fehlner, Thomas P. (2002). "Routes to Compounds Containing M−B Bonds. Reaction of [Cp*FeH2]2 with BH3·THF, Yielding the Hydrogen-Rich arachno-Ferrapentaborane 1-Cp*FeB4H11 (Cp* = η5-C5Me5)". Organometallics 21 (14): 2821–2823. doi:10.1021/om020273y.
- ↑ 2.0 2.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Grimes, Russell N. (1982). Metal Interactions with Boron Clusters. ISBN 9780306409332.
- ↑ Kang, H. C.; Lee, S. S.; Knobler, C. B.; Hawthorne, M. F. (1991). "Syntheses of Charge-Compensated Dicarbollide Ligand Precursors and Their Use in the Preparation of Novel Metallacarboranes". Inorganic Chemistry 30 (9): 2024–2031. doi:10.1021/ic00009a015.
- ↑ Sivaev, I. B.; Bregadze, V. I. (2000). "Chemistry of Nickel and Iron Bis(dicarbollides). A Review". Journal of Organometallic Chemistry 614-615: 27–36. doi:10.1016/S0022-328X(00)00610-0.
- ↑ Grimes, R. N. (1982). "Metallacarboranes and Metallaboranes". Comprehensive Organometallic Chemistry. pp. 459–542. doi:10.1016/B978-008046518-0.00009-X. ISBN 9780080465180.
 |


